A new study, recently published in Nature Communications, unveils a powerful new method for studying the inner workings of cell nuclei during embryonic stem cell differentiation.
The team led by Dr. Eitan Lerner from the Institute of Life Sciences and the Center for Nanoscience and Nanotechnology and Prof. Eran Meshorer, from the Institute of Life Sciences and the Edmond and Lily Safra Center for Brain Sciences (ELSC) at The Hebrew University of Jerusalem and Prof. Sarah Rauscher from the University of Toronto used a specific feature of special glowing proteins (fluorescent proteins or FPs), their fluorescence lifetimes, to learn about how cells change and grow.
These protein-based reporters helped them understand how parts of DNA called heterochromatin are packed inside the cell nucleus and what happens to these parts during cell development. This discovery gives us important information about how our cells work and change over time.
Fluorescent proteins have long been a staple in cellular research, providing scientists with a window into the inner world of cells. However, this study conducted introduces an innovative application of FPs, allowing them to serve as indicators of local density within bio-condensates in the cell nucleus.
By observing that common monomeric fluorescent proteins (FPs) exhibit lower fluorescence lifetimes in certain conditions, the researchers established a connection between this light behavior and higher levels of crowding within cellular structures. This innovative use of fluorescence provides valuable insights into cellular biology, allowing scientists to investigate how local densities are distributed within critical cellular components like heterochromatin protein 1α (HP1α).
The study zeroed in on mouse embryonic stem cells (ESCs) and their transition during early differentiation. Initially, the researchers observed a significant variation in the way heterochromatin proteins were packed within cellular structures called HP1α condensates in pluripotent ESCs.
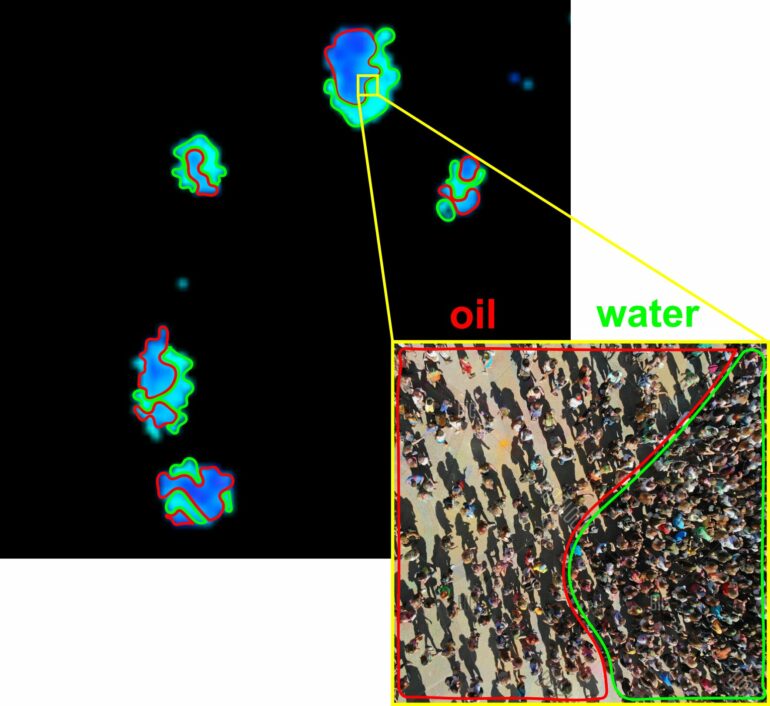
This complexity defied a simple explanation, suggesting it wasn’t like a single liquid phase, but rather like multiple different liquid phases with different densities separated from each other, much like what happens in different areas inside a crowd of people. However, as the cells began to differentiate and mature, a notable transformation occurred. The contents within these structures became more evenly distributed, resembling the behavior of a liquid, albeit a complex one.
Key findings
Fluorescent proteins can act as indicators, revealing how closely packed materials are in heterochromatin within structures of the cell nucleus. Initially, embryonic stem cells (ESCs) displayed a varied distribution of tightly packed materials within these structures, much like the different densities in a crowd of people or between an oil droplet and its surrounding water. However, as these cells began to differentiate and mature, the contents within these structures became more uniformly distributed, resembling the behavior of a single liquid phase.
Commenting on the study, Prof. Eran Meshorer stated, “Our research opens new doors for understanding the complexities of cellular behavior during differentiation. The ability to precisely track local densities within bio-condensates using fluorescent proteins provides valuable insights into cellular development that were previously hidden from view.”
Co-author Dr. Eitan Lerner added, “This breakthrough method offers researchers a powerful tool for investigating the intricate processes that underlie critical cellular events in general and stem cell differentiation in particular. This discovery paves the way for gaining better understanding of the inner cell intricacies, and as such has the potential to reshape our understanding of cell biology.”
This research represents a significant step forward in the field of cellular biology and holds promise for future applications in understanding various cellular processes and diseases.
More information:
Khalil Joron et al, Fluorescent protein lifetimes report densities and phases of nuclear condensates during embryonic stem-cell differentiation, Nature Communications (2023). DOI: 10.1038/s41467-023-40647-6
Provided by
Hebrew University of Jerusalem
Citation:
New fluorescent approach reveals different DNA densities in stem cells (2023, September 19)