The function of the glycolytic enzyme hexokinase 1 differs depending on its location within the cell, according to a Northwestern Medicine study published in Molecular Cell.
The enzyme uses different pathways to regulate glucose processing or immune response, determined by whether it’s bound to mitochondria or in the cytoplasm.
“The glycolytic function of this enzyme has been known about for decades, but we showed this function is dependent on the cellular localization of this enzyme,” said Hossein Ardehali, MD, Ph.D., the Thomas D. Spies Professor of Cardiac Metabolism, director of the Center for Molecular Cardiology at the Feinberg Cardiovascular and Renal Research Institute and the Medical Scientist Training Program (MSTP), and senior author of the study.
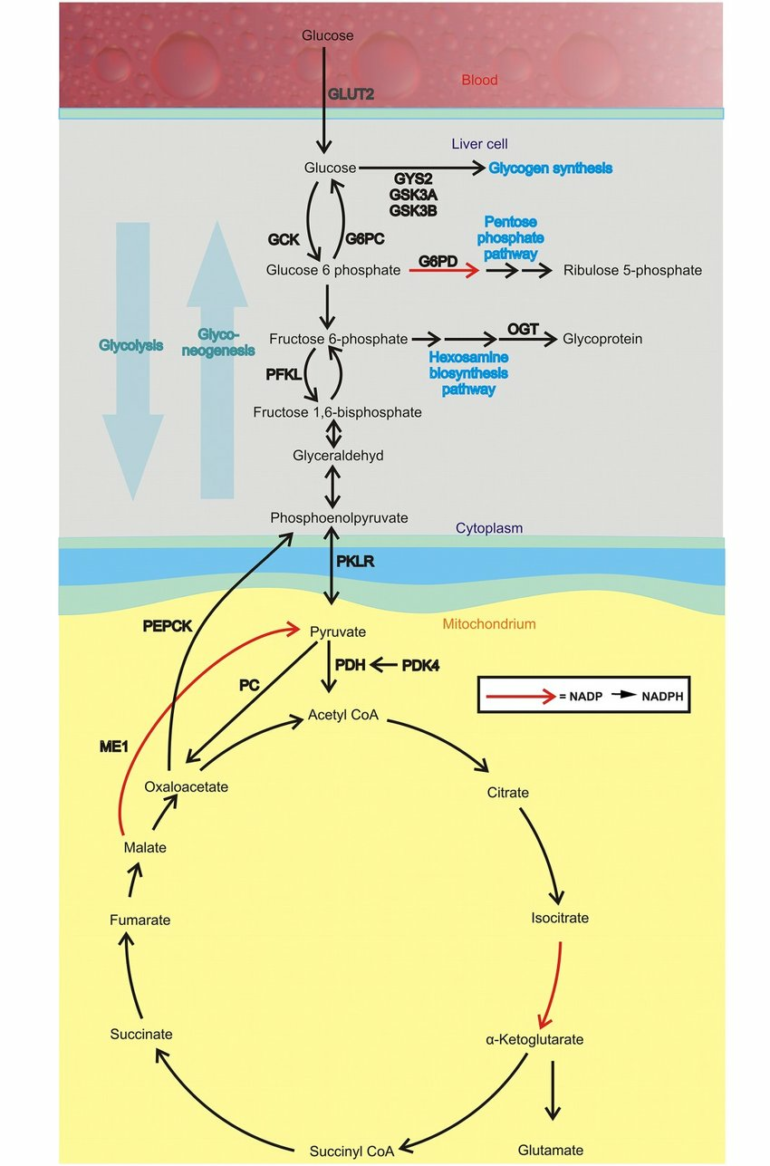
Glucose is a critical molecule for all cells, fueling a variety of processes including glycolysis. When glucose first enters a cell, it must be “processed” by a hexokinase before it can be used, similar to processing raw sugarcane before it can be turned into different products.
“After glucose is processed by hexokinase, it’s committed to being utilized by the cell in a variety of different ways,” said Adam De Jesus, a student in the Driskill Graduate Program in Life Sciences (DGP) and lead author of the study.
Most hexokinase is bound to the mitochondria, preparing glucose for glycolysis, but hexokinases are also present in in the cytosol, the liquid-filled interstitial space between organelles within a cell. The specific function of cytosolic hexokinase was poorly understood, so Ardehali, De Jesus and their collaborators studied the effects of removing mitochondrial binding domains from hexokinase in mice macrophage cells—forcing hexokinase into the cytosol of these cells.
Giving these mice a large dose of a lipopolysaccharide—a cellular component found on bacteria—resulted in harmful inflammation. Through multiple rounds of experiments, the investigators discovered that cytosolic hexokinase engages an entirely different pathway compared to mitochondrial hexokinase.
“We found the exclusive activation of this pathway creates a bottleneck, so you have this accumulation of metabolites upstream,” De Jesus said. “Instead, these metabolites are shunted to other pathways such as the pentose phosphate pathway, which is very important in regulating inflammation.”
The discovery of an entirely new function of a well-known enzyme based on its location raises questions about its effects in other cell types, such as T-cells and neurons, according to Ardehali.
“Just displacing it from the mitochondria to the cytoplasm gives you an entirely different function,” Ardehali said. “How it affects cellular function in cells besides macrophages is the focus of the current studies in our lab.”
More information:
Adam De Jesus et al, Hexokinase 1 cellular localization regulates the metabolic fate of glucose, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.02.028
Provided by
Northwestern University
Citation:
New function for glucose metabolism enzyme (2022, March 23)