Since its breakthrough development more than a decade ago, CRISPR has revolutionized DNA editing across a broad range of fields. Now scientists are applying the technology’s immense potential to human health and disease, targeting new therapies for an array of disorders spanning cancers, blood conditions and diabetes.
In some designed treatments, patients are injected with CRISPR-treated cells or with packaged CRISPR components with a goal of repairing diseased cells with precision gene edits. Yet, while CRISPR has shown immense promise as a next-generation therapeutic tool, the technology’s edits are still imperfect. CRISPR-based gene therapies can cause unintended but harmful “bystander” edits to parts of the genome, at times leading to new cancers or other diseases.
Next-generation solutions are needed to help scientists unravel the complex biological dynamics behind both on- and off-target CRISPR edits. But the landscape for such novel tools is daunting, since intricate bodily tissues feature thousands of different cell types and CRISPR edits can depend on many different biological pathways.
University of California San Diego researchers have developed a new genetic system to test and analyze the underlying mechanisms of CRISPR-based DNA repair outcomes. As described in Nature Communications, Postdoctoral Scholar Zhiqian Li, Professor Ethan Bier and their colleagues developed a sequence analyzer to help track on- and off-target mutational edits and the ways they are inherited from one generation to the next. Based on a concept proposed by former UC San Diego researcher David Kosman, the Integrated Classifier Pipeline (ICP) tool can reveal specific categories of mutations resulting from CRISPR editing.
Developed in flies and mosquitoes, the ICP provides a “fingerprint” of how genetic material is being inherited, which allows scientists to follow the source of mutational edits and related risks emerging from potentially problematic edits.
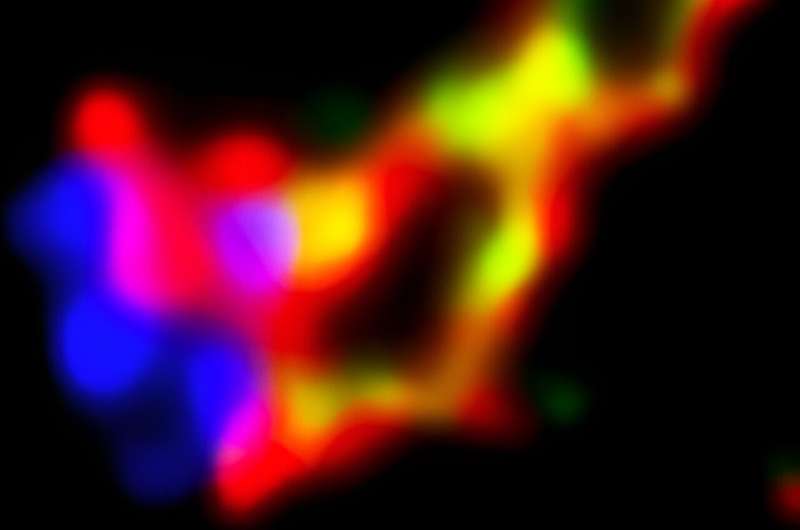
UC San Diego researchers have created a new system that reveals specific categories of potentially risky mutations resulting from CRISPR edits. This high magnification image reveals CRISPR-based DNA transcription of the homothorax gene in a fruit fly embryo. © Bier Lab, UC San Diego
“The ICP system can cleanly establish whether a given individual insect has inherited specific genetic components of the CRISPR machinery from either their mothers or fathers since maternal versus paternal transmission result in totally different fingerprints,” said Bier, a professor in the UC San Diego School of Biological Sciences.
The ICP can help untangle complex biological issues that arise in determining the mechanisms behind CRISPR. While developed in insects, ICP carries vast potential for human applications.
“There are many parallel applications of ICP for analyzing and following CRISPR editing outcomes in humans following gene therapy or during tumor progression,” said study first author Li. “This transformative flexible analysis platform has many possible impactful uses to ensure safe application of cutting-edge next-generation health technologies.”
ICP also offers help in tracking inheritance across generations in gene drive systems, which are new technologies designed to spread CRISPR edits in applications such as stopping the transmission of malaria and protecting agricultural crops against pest destruction.
For example, researchers could select a single mosquito from the field where a gene-drive test is being conducted and use ICP analysis to determine whether that individual had inherited the genetic construct from its mother or its father, and whether it had inherited a defective element lacking the defining visible markers of that genetic element.
“The CRISPR editing system can be more than 90% accurate,” said Bier, “but since it edits over and over again it will eventually make a mistake. The bottom line is that the ICP system can give you a very high-resolution picture of what can go wrong.”
In addition to Li and Bier, co-authors included Lang You and Anita Hermann. Prior Bier lab member Kosman also made important intellectual contributions to this project.
More information:
Zhiqian Li et al, Developmental progression of DNA double-strand break repair deciphered by a single-allele resolution mutation classifier, Nature Communications (2024). DOI: 10.1038/s41467-024-46479-2
Provided by
University of California – San Diego
Citation:
New genetic analysis tool tracks risks tied to CRISPR edits (2024, March 26)