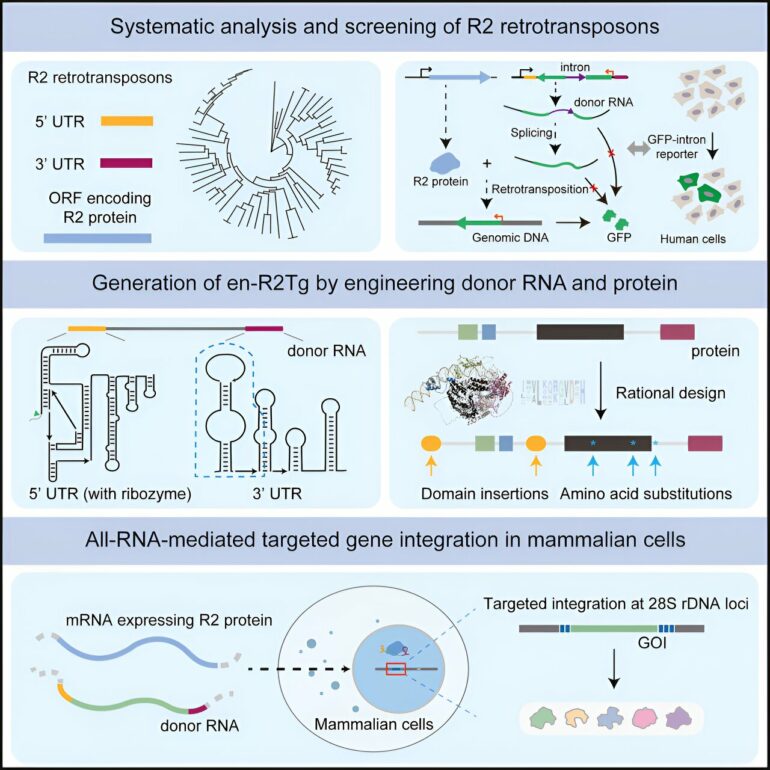
In a recent study published in Cell, a research team led by Li Wei and Zhou Qi from the Institute of Zoology of the Chinese Academy of Sciences has developed an innovative gene-writing technology based on retrotransposons. This achievement enables all-RNA-mediated targeted gene integration in human cells.
Efficient and precise integration of gene-sized DNA remains a major challenge in genome engineering. Current technologies rely primarily on DNA templates as donors for gene integration. However, DNA donors face numerous challenges in practical biomedical applications, such as high immunogenicity, difficulty in in vivo delivery, and the risk of random integration into the genome.
On the other hand, RNA donors have lower immunogenicity compared to exogenous DNA donors, which can be effectively delivered using non-viral vectors, and are rapidly degraded in cells without the risk of random integration—thus addressing many of the challenges associated with DNA donors. However, there are currently very few technologies that can use RNA donors to achieve targeted gene-sized DNA integration in human cells.
R2 retrotransposons are mobile elements that use RNA intermediates to specifically integrate into the host 28S rDNA genomic site. There are 219 copies of this site present in the human genome, located away from protein-coding genes—making it a “safe harbor” suitable for gene integration. Despite its discovery in the 1980s, its potential use for integrating large-fragment genes into human cells has not been fully explored.
In this study, through systematic analysis and screening, the researchers identified the avian genome-derived R2Tg system as active in human cells, albeit with low efficiency. Using several engineering approaches, the researchers created an optimized version of R2Tg, en-R2Tg.
Delivered by lipid nanoparticle (LNP), a non-viral vector used in clinic, the en-R2Tg gene-writing tool can achieve 25% gene integration efficiency in human liver cells, and it enables over 60% site-specific gene integration efficiency in mouse embryos.
In addition, the en-R2Tg system exhibits high gene integration specificity at the 28S rDNA safe harbor site. This helps to minimize the risk of mutagenesis caused by random gene integration generated by technologies such as retroviruses.
In summary, this study develops an all-RNA-mediated, efficient, and precise gene-writing technology based on naturally occurring R2 retrotransposons.
“This technology opens a door for the development of novel gene therapeutics. When it comes to a disease-related gene, there can be many different mutations that cause the same disease. Our technology offers a more general approach in which we can integrate a normal gene directly into the genome to restore function, regardless of the type of mutation,” said Li Wei, corresponding author of the study.
“We may even be able to use LNP to deliver our gene-writing tool and create CAR-T cells directly in our bodies to treat cancer. This could make the whole process as easy as getting a vaccination. We’re excited about the potential for further development and application of this new technology in the future,” said Li.
More information:
Yangcan Chen et al, All-RNA-mediated targeted gene integration in mammalian cells with rationally engineered R2 retrotransposons, Cell (2024). DOI: 10.1016/j.cell.2024.06.020
Provided by
Chinese Academy of Sciences
Citation:
Novel gene writing technology enables all-RNA-mediated targeted gene integration in human cells (2024, July 12)