Efficient communication between cells is vital for many biological processes, such as recruiting immune cells to combat disease. The rapid release of at least 80 signaling molecules, including tumor necrosis factor and epidermal growth factor, is controlled by a membrane-bound protease called ADAM17.
This process involves ADAM17 cleaving (shedding) the signaling molecules from their precursor forms in the membrane of a cell. The “pseudoprotease” iRhom2 aids in the maturation and transport of ADAM17. A lack of structural insight, however, has left the mechanisms underlying this process unclear.
Published today in Molecular Cell, scientists from St. Jude Children’s Research Hospital and the University of Oxford have used cryo-electron microscopy to reveal a series of structures of the human ADAM17/iRhom2 complex in both the active and inactive states. These structures show that iRhom2 acts as a gatekeeper to the ADAM17 lifecycle, interacting with key regions of ADAM17 that control its activity.
The work also offers potential avenues for drug design to combat diseases of chronic inflammation and autoimmune dysregulation. “We think there is potential to design small molecules that target iRhom2 instead of ADAM17 directly, or the interface between iRhom2 and ADAM17,” said co-corresponding author, Chia-Hsueh Lee, Ph.D., St. Jude Department of Structural Biology.
Defunct protease finds a new lease on life
iRhom2 is a pseudoprotease: It has all the structural features of a protease from the rhomboid family, except it lacks the protease-defining ability to cleave proteins. However, like an old dog learning new tricks, iRhom2 plays new-found roles in the scaffolding and transport of ADAM17.
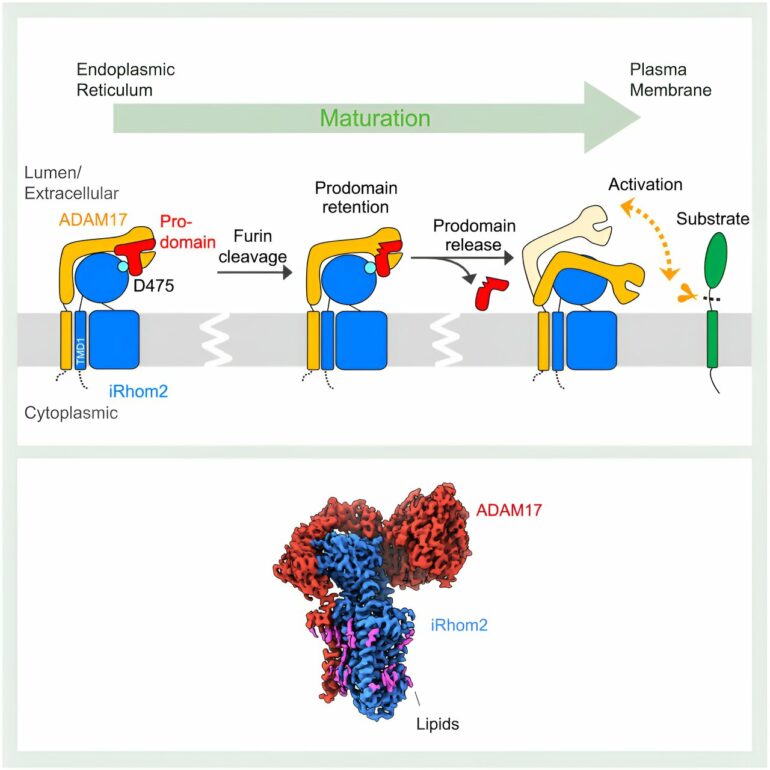
As structural studies revealed, iRhom2’s impact on ADAM17 function starts during the initial synthesis of the two proteins. “The endoplasmic reticulum is where ADAM17 and iRhom2 are made, and at this stage, they form a complex,” explained Lee. iRhom2 then facilitates shuttling of ADAM17 to the Golgi apparatus for maturation, where the previous concept of iRhom2 serving merely as a “passive scaffold” was put to the test by Lee’s structures.
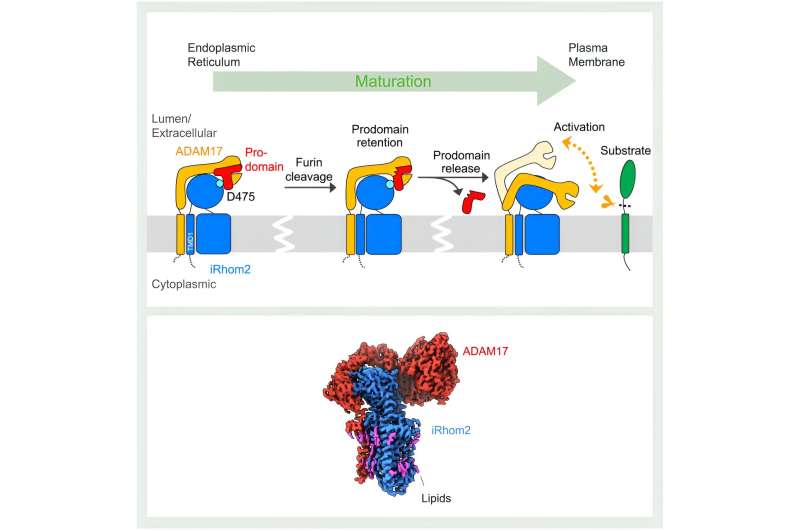
Graphical abstract. © Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.04.025
The interaction between iRhom2 and ADAM17 was presumed to occur exclusively between the proteins’ transmembrane domains, which pin the proteins to the cell membrane. To the researchers’ surprise, the two proteins also interact through their extracellular regions. Through those interactions, iRhom2 holds onto an important region of ADAM17 called the prodomain. The prodomain is a region that acts as a safety pin on a fire extinguisher.
“ADAM17 itself is self-inhibited. It has a prodomain cap to block its own protease site,” explained Lee.
iRhom2 helps pop the cap on ADAM17 activity
Within the Golgi apparatus, this self-regulating prodomain cap is cleaved. However, iRhom2 retains the ADAM17 cap until the complex reaches the cell membrane. This step is crucial to prevent the premature activation of ADAM17. The new structures reveal that once the cap is removed, ADAM17 is activated. This activation is marked by a significant gain in flexibility, which allows ADAM17 to search its surrounding space for substrates.
ADAM17 has been shown to play a role in the development and progression of multiple cancer types; however, the ADAM family of proteins is well conserved. A drug which targets ADAM17 can lead to off-target effects. Lee is optimistic that the structures can offer a blueprint for drug design ingenuity.
“Based on our model, if we could hold these two proteins together, for example, through a binder that enhances the interaction between iRhom2 and ADAM17, then iRhom2 will not release the prodomain cap,” said Lee. “This will significantly reduce ADAM17 activity.”
The study’s first authors are Fangfang Lu, University of Oxford and Hongtu Zhao, St. Jude. The study’s other co-corresponding authors are Hongtu Zhao, St. Jude and Matthew Freeman, University of Oxford. The study’s additional authors are Yaxin Dai and Yingdi Wang, St. Jude.
More information:
Fangfang Lu et al, Cryo-EM reveals that iRhom2 restrains ADAM17 protease activity to control the release of growth factor and inflammatory signals, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.04.025
Provided by
St. Jude Children’s Research Hospital
Citation:
Repurposed protease controls important signaling molecule-activating protein (2024, May 22)