Biosensors—devices that use biological molecules to detect the presence of a target substance—have enormous potential for detecting disease biomarkers, molecules-in-action in diverse biological processes, or toxins and other harmful substances in the environment.
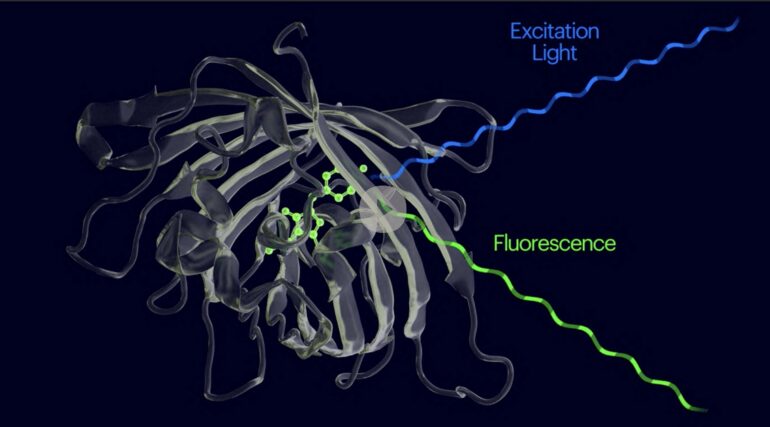
One of the more common types, fluorescent biosensors, consists of a target-binding biomolecule attached to a probe molecule that emits fluorescent light. However, fluorescent biosensors are typically low-contrast reagents because their fluorescent probes are always “on,” and un-bound biosensor molecules need to be washed away before an accurate signal can be detected.
A major step forward are high-contrast “binding-activated fluorescent biosensors” (nanosensors) that only light up when they bind to their target molecule, but creating such nanosensors is challenging as effective target-binding and a fluorescence on-switch need to be combined in a small molecular package that also can be efficiently delivered to various types of samples, and cost-efficiently manufactured at scale.
Now, a collaborative research team at the Wyss Institute at Harvard University, Harvard Medical School (HMS), MIT, and the University of Edinburgh, UK, has developed a synthetic biology platform to streamline the discovery, molecular evolution, and cost-effective manufacturing of small and highly efficient nanosensors that can detect specific proteins, peptides, and small molecules by increasing their fluorescence up to 100-fold in less than a second.
As a key component, the platform uses new fluorogenic amino acids (FgAAs) that can be encoded into target-binding small protein sequences (binders) with the help of an innovative methodology that enables the in vitro expansion of the genetic code.
Through a process of high-throughput sensor screening, validation, and directed evolution, the platform enables the rapid and cost-effective transformation of protein binders into high-contrast nanosensors for a wide range of applications in fundamental research, environmental monitoring, medical diagnostics and augmented therapeutics. The findings are published in Nature Communications.
“We have long worked on expanding the genetic code of cells to endow them with new capabilities to enable research, biotechnology and medicine in different areas, and this study is a highly promising extension of this endeavor in vitro,” said Wyss Core Faculty member George Church, Ph.D., who led the study.
“This novel synthetic biology platform solves many of the obstacles that stood in the way of upgrading proteins with new chemistries, as exemplified by more capable instant biosensors, and is poised to impact many biomedical areas.”
Church is a leader of the Wyss Institute’s Synthetic Biology Platform, and also the Robert Winthrop Professor of Genetics at HMS and Professor of Health Sciences and Technology at Harvard University and MIT.
Protein plus scaffold equals nanosensor
The team, spearheaded by co-first and co-corresponding author Erkin Kuru, Ph.D. in Church’s group, built on the previous discovery that FgAAs could convert known protein binders into optical sensors whose fluorescence is switched on when their FgAA is sandwiched between their binder sequence and the target molecule.
The Wyss researchers collaborated with co-corresponding author Marc Vendrell, Ph.D., a Professor at the University of Edinburgh and expert in translational chemistry and biomedical imaging on the study with whom Kuru shared an early interest in FgAAs.
Starting out in the pandemic, the team first envisioned an “instant COVID-19 diagnostic” and focused on a miniature engineered antibody (nanobody) that binds to the SARS-CoV-2 Spike protein on the virus’s surface.
They created hundreds of variants of the binder in which they essentially assembled FgAAs by chemically linking cysteine or lysine amino acids that were genetically introduced to positions known to be in close contact with the Spike target to one of 20 different chemical fluorogenic scaffolds.
Using a simple binding assay, they selected the fluorogenic variants that produced the highest increases in fluorescence within milliseconds upon target-binding.
They then used the same process to engineer nanosensors from nanobodies or mini-proteins that bind to different SARS-CoV-2 target sites, as well as to a range of other molecular targets, including the cancer-relevant cellular growth factor receptor EGFR, the ALFA-tag peptide used by cell biologists to track proteins within cells, and the stress hormone cortisol.
Importantly, the nanosensors also effectively signaled the presence of their targets in human cells and live bacteria under the microscope, demonstrating their utility as effective imaging tools.
Nanoensor evolution
Despite its potential, the first version of the platform was limited by relying on a labor- and time-intensive process involving multiple purification steps of the produced binder sequences. “We wanted to expand our molecular design space much further by increasing the platform’s high-throughput capabilities,” said Kuru.
“To achieve this, we enabled the ribosome, which naturally synthesizes all proteins in cells, to do most of the work in an engineered cell-free process.”
In the 2.0 version of their platform, the team pre-fabricated a so-called “synthetic amino acid” with a fluorogenic scaffold already pre-attached to it. Synthetic amino acids already have proven their value in therapeutics such as the weight-loss drug Ozempic; however, they cannot be easily incorporated into protein sequences because there is no natural machinery for them to be handled by the ribosome.
“To overcome this obstacle, we reassigned a rarely used codon in the universal genetic code with the help of a new genetic expansion chemistry, so that it could encode synthetic amino acids like our pre-fabricated non-standard FgAAs.
Essentially, we retrofitted the protein synthesis process for the construction of binding-activated fluorescent nanosensors,” said co-first author Jonathan Rittichier, Ph.D., who co-developed the method.
Their new process not only enabled the researchers to produce millions of nanosensor candidates at a time, but also helped accelerate the subsequent testing of the nanosensors, as the entire synthesis mix could be directly combined with the target molecule or added to living cells without any additional purification.
They can now investigate hundreds of variants in a day rather than a few dozen over several weeks. Highlighting the advanced platform’s power, they discovered a specific position to encode their FgAAs in the original SARS-CoV-2 nanobody binder that, unexpectedly, resulted in a higher-affinity nanosensor than their original COVID-19 nanosensor upon contacting the Spike target protein.
Finally, as this would significantly increase the potential to create superior nanosensors, the team leveraged their platform to optimize the nanobody sequence itself. They took advantage of a classical synthetic biology process known as “directed evolution” in which proteins are optimized through iterative design-build-test cycles that use versions of a protein with maximum capabilities identified in one cycle as the basis to find even better ones in the following one.
Starting with the best nanosensor that they had previously engineered to instantly detect the original SARS-CoV-2 strain’s Spike protein, Kuru, Rittichier, and the team created expansive nanobody libraries encompassing variants that kept the non-standard FgAA at the original position but had many standard amino acids at other critical positions substituted with structurally different ones.
Evolving the best of them further led them to new nanosensors with orders of magnitude higher binding affinities toward the Spike protein. Interestingly, by using a tweaked version of this directed evolution system, they discovered nanosensors that were able to selectively detect distinct newer omicron variants.
“This is an important step forward in our capabilities to quickly design low-cost fluorescent biosensors for real-time disease monitoring and with huge potential for diagnostics and precision medicine,” said Vendrell.
Kuru added, “we can also incorporate synthetic amino acids with many other functionalities into all kinds of proteins to create new therapeutics, and a much broader range of research tools.”
Indeed, Kuru and co-authors Helena de Puig, Ph.D. and Allison Flores, along with Church and senior author and Wyss Core Faculty member James Collins, Ph.D., have also embarked on the Wyss Institute’s AminoX project, which leverages the platform to develop new therapies.
“This highly innovative work enabling a new and more powerful generation of binding-activated biosensors demonstrates the remarkable powers of synthetic biology.
“The Wyss team succeeded in engineering a fundamental biological process into a platform with vast potential for ultimately solving many diagnostic and therapeutic problems,” said Wyss Founding Director Donald Ingber, M.D., Ph.D., who also is also the Judah Folkman Professor of Vascular Biology at HMS and Boston Children’s Hospital, and the Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS.
Additional authors on the study are Subhrajit Rout, Isaac Han, Abigail Reese, Thomas Bartlett, Fabio De Moliner, Sylvie Bernier, Jason Galpin, Jorge Marchand, William Bedell, Lindsay Robinson-McCarthy, Christopher Ahern, Thomas Bernhardt, and David Rudner.
More information:
Erkin Kuru et al, Rapid discovery and evolution of nanosensors containing fluorogenic amino acids, Nature Communications (2024). DOI: 10.1038/s41467-024-50956-z
Provided by
Harvard University
Citation:
Researchers develop molecular biosensors that only light up upon binding to their targets (2024, September 5)