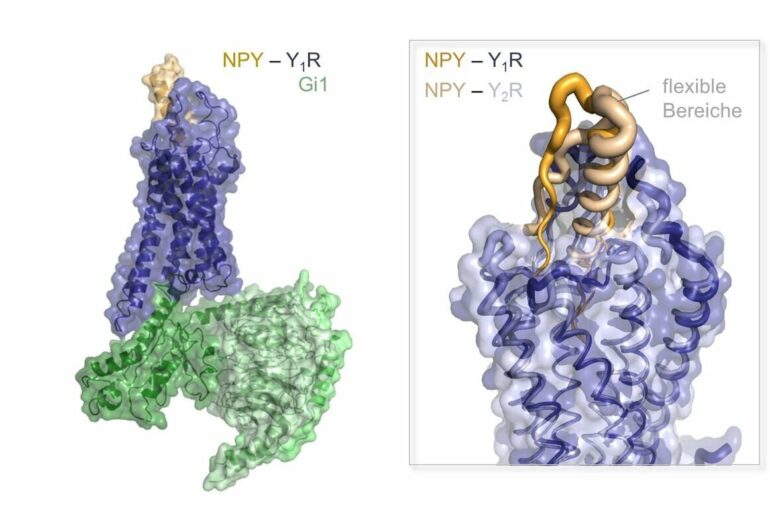
The human body consists of trillions of cells that constantly communicate with one another. A central role in this communication process is played by receptor proteins on the cell surface. Since they often serve as drug targets, they have been the subject of intensive research. Often there are whole families of receptors. The signal messengers as well as the receptors are very similar, so it is not clear how the signals are distinguished from one another at the molecular level. Now, in a joint research project, scientists from Collaborative Research Centre 1423 at Leipzig University, the Hangzhou Institute for Advanced Study and the Chinese Academy of Sciences in Shanghai have succeeded in determining high-resolution structures for three related signaling complexes that occur naturally in the body for the neuropeptide Y (NPY) receptor family, thus shedding light on the small but essential differences. The researchers have now published their new findings in the journal Science Advances.
The NPY family consists of a total of three related peptide ligands: NPY, PP and PYY, which have different functions in the body. These act as messengers locally in the tissues, especially in the brain, and via the bloodstream. They bind to four different receptors (Y1R, Y2R, Y4R and Y5R), with different combinations of peptide ligand and receptor occurring in different situations: while NPY in conjunction with Y1R signals hunger in the brain, PP bound to Y4R conveys a strong satiety signal. NPY receptors are also of interest for modern cancer therapies. A high number of Y1R is characteristic for breast cancer cells, which is why NPY variants that selectively bind only to this receptor could be used to deliver drugs specifically to these cells. Healthy breast tissue, on the other hand, contains mainly the receptor Y2R. It would make sense to bypass this in order to spare the healthy tissue.
To be able to develop targeted active substances, it is therefore highly important to know the molecular blueprint of these complexes and the underlying regulatory mechanisms. In addition to the molecular structures visualized by Professor Qiang Zhao from the Hangzhou Institute for Advanced Study and Professor Beili Wu of the Chinese Academy of Sciences using cryogenic electron microscopy, Professor Annette Beck-Sickinger and Dr. Anette Kaiser of Leipzig University conducted biochemical studies that shed more light on the complex mechanisms that bind the peptides to their receptors and supported the results of the structural studies. It was possible to find the relevant regions in the peptides and receptors in the complex.
The working groups have been conducting joint research in this field for more than ten years, and these new results build on extensive preliminary work. This makes this joint publication—the third by the working groups—all the more valuable. This is because a novel test system showed that the peptides use different “docking pathways” and that this can lead to different signals in the cell. The flexibility and mobility of the complexes in certain areas also plays an important role. Professor Annette Beck-Sickinger says, “Some of the flexibility of the peptide and receptor is thus retained even in the bound state. The causes and consequences of this are now being further investigated in ongoing studies in CRC 1423, as is the question of what other factors influence the recognition between peptides and receptors.”
More information:
Tingting Tang et al, Receptor-specific recognition of NPY peptides revealed by structures of NPY receptors, Science Advances (2022). DOI: 10.1126/sciadv.abm1232
Provided by
Leipzig University
Citation:
Researchers discover molecular mechanisms of signal recognition in the neuropeptide system (2022, May 6)