Prime editing technologies allow scientists to precisely edit the genome in a variety of ways and could one day be used to treat genetic diseases. Now researchers at the Broad Institute of MIT and Harvard have used cutting-edge continuous laboratory evolution and engineering methods to develop improved versions of the gene-editing tool.
Their new editors are more efficient and specialized than previous versions, and are able to modify DNA in cultured cells and in animals that have been difficult to edit, including in immune system cells and inside the brain. The editing molecules are also smaller, potentially making it easier to deliver them into cells in key parts of the body as new disease treatments.
Prime editing can make targeted insertions, deletions and other changes to DNA, and was first described in 2019 by the lab of David Liu, core institute member, Richard Merkin Professor, and director of the Merkin Institute of Transformative Technologies in Healthcare at the Broad Institute.
The technique combines multiple molecular machines: a disabled Cas9 protein that can nick DNA; an engineered prime editing guide RNA (pegRNA) that both specifies the location of the edit and also contains new genetic instructions to install at that location; and an engineered version of an enzyme called reverse transcriptase that uses that RNA as a template to make specific changes to the DNA.
In the past, Broad researchers have optimized the pegRNA and the way the cell responds to prime editing to boost editing efficiency. In the new work, they focused on improving the heart of the prime editing system—the reverse transcriptase. Their efforts have yielded a suite of new prime editors, each evolved in the lab to specialize in different editing tasks. In a paper published August 31 in Cell, the team unveils the prime editing systems, dubbed PE6a through PE6g, each containing a new reverse transcriptase or Cas9 variant.
The new prime editors are two to 20 times more efficient than previous ones, making them potentially more useful as therapeutics. The researchers are already using the PE6 editors in nearly all of their prime editing projects to edit a wide range of cell types, including in animals and in therapeutically relevant cultured cells.
“We’re really excited to share these state-of-the-art prime editing tools with the community,” said Liu, who is also a professor at Harvard University and a Howard Hughes Medical Institute investigator. “We hope these new prime editors will become an integral part of the gene editing field, which has created so much promise and progress in the life sciences and in medicine.”
Jordan Doman and Smriti Pandey, graduate students in the Liu lab, are co-first authors of the study. They note that this new study began in the summer of 2019, before the Liu lab even reported the first prime editing system.
“The early days of this project focused on figuring out how this complicated molecular machine was working and what its preferences were. Then, more recently, we worked on turning this enhanced understanding into improved tools and potential therapeutics,” said Doman.
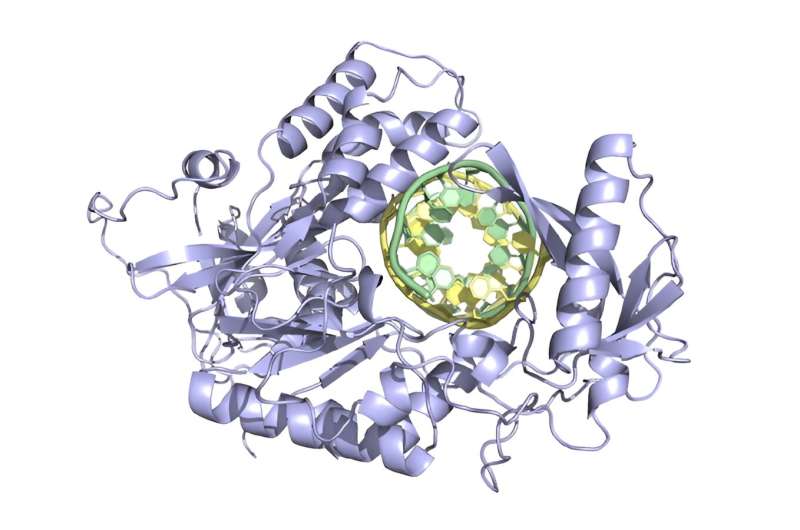
Structure (predicted by AlphaFold) of a new prime editor developed by the David Liu lab. © Broad Institute of MIT and Harvard
Optimizing every component
Prime editing promises to correct most disease-causing genetic mutations, but its efficiency can vary widely depending on the location and type of edit and on the type of cells being edited. In some cases, only a few percent of cells exposed to a prime editor end up with the desired genetic edit.
To improve this efficiency for therapeutic applications, Liu’s team has focused on studying and improving every part of the complex prime editing system.
“We had previously optimized every other component of the prime editing machinery,” Liu said. “What had not been done until now is improving the reverse transcriptase in a way that’s tailor-made for prime editing.”
Reverse transcriptase proteins that copy RNA templates into strands of DNA are found naturally in all plant and animal cells and in many viruses. But naturally occurring reverse transcriptases generally result in poor prime editing efficiency, and researchers had not previously reported reverse transcriptases that consistently increase the editing efficiency of prime editors beyond that of the engineered reverse transcriptase used in the original prime editing system.
To optimize the reverse transcriptase domain of prime editors in a more directed way, Liu’s group turned to PACE (phage-assisted continuous evolution), a directed evolution approach developed by Liu in 2011. The method involves growing a diversity of rapidly evolving bacteriophages—the viruses that infect bacteria—in laboratory “lagoons” that undergo continuous dilution. Only those expressing gene variants with specified desirable traits can survive by replicating faster than they are diluted.
In the new work, the scientists developed a PACE system that imposed a Darwinian selection for bacteriophages that could carry out prime editing to correct a defective bacteriophage gene. Over hundreds of hours, the bacteriophages encoding the prime editors underwent thousands of generations of evolution, rapidly generating new reverse transcriptases that more efficiently make edits.
Liu’s team challenged the PACE system with different types of prime editing tasks, such as inserting short or long stretches of DNA, and then tested the editors that survived. Surprisingly, the researchers found that evolved prime editors that improved editing efficiency in one task, such as adding or substituting just a few letters of DNA, performed poorly in other contexts, such as adding long stretches of DNA.
“It turned out that PACE evolved these prime editors to specialize in exactly the kind of edits that we demanded they make during PACE,” Liu said. “But an editor that worked best for one edit didn’t work best for another. This discovery disproves the assumption that one type of prime editor can optimally perform any kind of prime edit.”
Toward therapeutics
As the team tested the PACE-evolved editors in different scenarios, they revealed a set of rules dictating when each of the seven prime editing systems should be used, and then did more experiments to demonstrate their utility.
For example, they showed that some PE6 variants can insert longer stretches of DNA ranging from 38 to 108 base pairs long in five different places in the genomes of a human cell line, with increased editing efficiency compared to current prime editors. These PE6 variants performed especially well in settings that have been challenging for researchers to edit, such as the brains of live mice.
“After years of optimization, we were excited to see PE6 variants expand the scope of prime editing,” said co-first author Pandey. “PE6 variants can particularly enhance editing at key therapeutically relevant sites that have been more challenging to target using previous systems.”
In one experiment, the researchers were able to insert a long sequence of DNA into 40% of the cells in the cortex of a mouse brain—a 24-fold improvement over the previous state-of-the art prime editing system.
“The difference between less than 2% editing efficiency and 40% editing efficiency can make the difference between something that’s an interesting research tool and something that can support a potential therapeutic application,” Liu said.
The new prime editors are also more compact than previous versions, allowing the entire prime editing platform to fit into some delivery systems that previous prime editors struggled to fit into. The streamlined prime editors may be easier to deliver in animal models and, eventually, human patients.
“The real litmus test of whether a technology is useful is whether people end up adopting it,” Liu said. “We’re excited to see how PE6 is applied to the prime editing field.”
More information:
Jordan L. Doman et al, Phage-assisted evolution and protein engineering yield compact, efficient prime editors, Cell (2023). DOI: 10.1016/j.cell.2023.07.039
Provided by
Broad Institute of MIT and Harvard
Citation:
Researchers make genome prime editors smaller and more efficient for therapeutic applications (2023, September 1)



