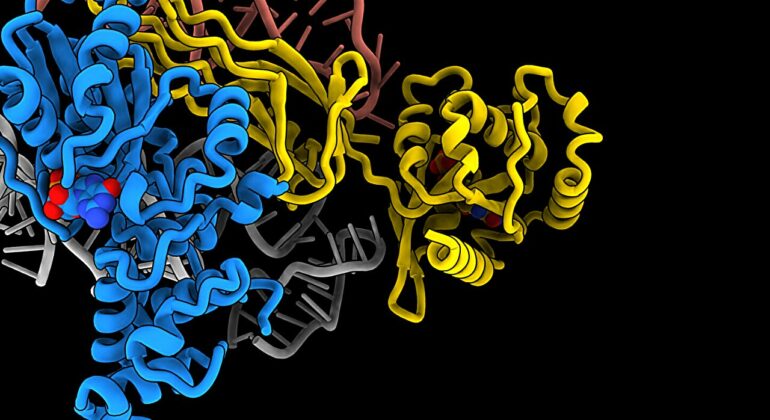
Human cells contain ribosomes, a complex machine that produces proteins for the rest of the body. Now the researchers have come closer to understanding how the ribosome works.
“It is amazing that we can visualize the atomic details of the ribosome. Because they are tiny—around 20–30 nanometers,” says Associate Professor Eva Kummer from the Novo Nordisk Foundation Center for Protein Research, who is responsible for the new study published in Nature Communications.
The ribosome is a part of the human cell consisting of ribosomal RNA and ribosomal proteins. It is like a factory that builds proteins by following a set of instructions inherent in the genes.
Ribosomes are found floating in the cell cytosol, cellular organelles such as mitochondria or the protoplasm of bacteria.
Using electron microscopy, Kummer and her colleagues Giang Nguyen and Christina Ritter have managed to produce a 3D model of a part of the human cell, the ribosome, which is no more than 30 nanometers in diameter.
More specifically, they have taken snapshots of how a ribosome is made.
“It is important to understand how the ribosome is built and how it works, because it is the only cell particle that produces proteins in humans and all other living organisms. And without proteins, life would cease to exist,” says Kummer.
Proteins are the primary building blocks of the human body. Your heart, lungs, brain and basically your whole body are made of proteins produced by the ribosome.
“From the outside, the human body looks pretty simple, but then consider the fact that every part of the body consists of millions of molecules, that are extremely complex, and that they all know what to do—that is pretty breathtaking,” says Kummer.
Folding, assembling, and moving to the right place
Before ribosomes can start to produce proteins, they first need to be assembled from more than 80 different components.
Kummer and her colleagues have obtained 3D models of three different stages of ribosome assembly. “It is a complex particle with lots of different parts—many proteins and RNA components—that must be folded, assembled, and moved to the right place. It does not all happen at once. Ribosome assembly is a gradual process involving several stages,” she explains.
Out of the three stages, the 3D model describing the earliest time point in the assembly is the most interesting, according to Kummer, as no one has been able to describe it before.
“At this stage, we can tell for example that a specific protein called GTPBP10 is eager to interact with a so-called RNA component that forms a long helix,” Kummer says. “In fact, towards the bottom of that helix is the catalytic center of the ribosome, which is where proteins are made. This is why it is so important that the helix is folded and placed correctly. “
To achieve this, GTPBP10 grabs the helix and puts it in the right position for protein synthesis.
This is just one of the many stages of ribosome assembly which the new study has shed light on—insight that may pave the way for more knowledge of various diseases.
“Errors in ribosome assembly severely reduce the capacity of our cells to make proteins. These are for example proteins that convert the energy from the food we eat into energy coins that the body can use to run all sorts of cellular processes.
“Now, if the mitochondrial ribosome does not work, our body cannot produce enough energy coins anymore and this leads to diseases such as neurodegenerative disorders and heart conditions. And during aging, the production of these energy coins also works less and less efficiently,” Kummer says. “The first step is understanding how things work. Only then can you try to change them.”
More information:
Thu Giang Nguyen et al, Structural insights into the role of GTPBP10 in the RNA maturation of the mitoribosome, Nature Communications (2023). DOI: 10.1038/s41467-023-43599-z
Provided by
University of Copenhagen
Citation:
Researchers produce 3D model of the ribosome and visualize how it is made (2024, February 23)