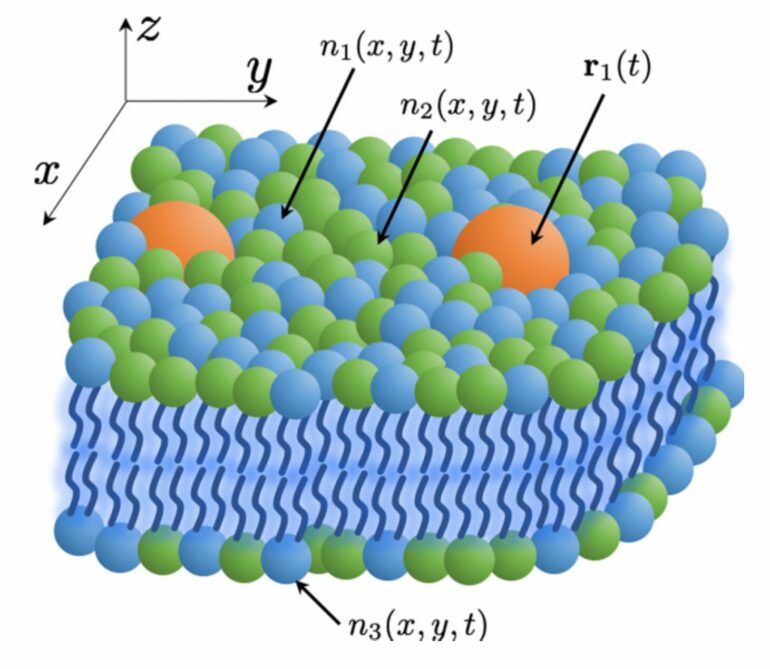
Lawrence Livermore National Laboratory scientists have developed a theoretical model for more efficient molecular-level simulations of cell membranes and their lipid-protein interactions, part of a multi-institutional effort to better understand the behavior of cancer-causing membrane proteins.
Developed under an ongoing collaboration by the Department of Energy (DOE) and the National Cancer Institute (NCI) aimed at modeling cell membrane interactions with RAS—a protein whose mutations are tied to about 30% of human cancers—the new model addresses a major problem in simulating RAS behavior, where conventional methods come up well short of reaching the time- and length-scales needed to observe biological processes of RAS-related cancers. The work appears in the latest issue of Physical Review Research.
“In order to carry out simulations across greater length and timescales, we developed a continuum model in which one can trade spatial resolution for computational efficiency,” said LLNL staff scientist and co-author Tomas Oppelstrup. “The novelty in this model lies primarily in being able to describe an arbitrary number of lipid species while also being derived directly from microscopic properties of the molecules, which can be computed from direct molecular simulations.”
The new model, based on Dynamic Density Functional Theory (DDFT), enables simulations that can access micron-level length-scales and timescales on the order of seconds, while maintaining resolution close to the current gold standard of molecular dynamics (MD) models. For perspective, these scales are hundreds of times larger in space and many thousands of times longer in time than those accessible with MD.
Co-authors said the publication shows that the DDFT framework is ideal for modeling multi-component cellular membranes as a continuum by incorporating the underlying molecular-level physics in a “rigorous and consistent way.”
“Continuum models in this field have been previously restricted to one or two lipid types and phenomenological descriptions of the lipid interactions,” said first author Liam Stanton, a San Jose State University professor and former staff scientist in LLNL’s Center for Applied Scientific Computing.
“The DDFT formalism has provided a pathway of maintaining molecular-scale accuracy for an arbitrary number of lipid types at vastly larger length- and timescales, inaccessible to MD simulations. At these scales, many new processes of biological relevance can be probed, and it will be exciting to see what this new tool will offer to cancer research and other biological communities.”
The macroscopic model described in the paper is a “clever combination of continuum dynamics and particle dynamics, and is constructed directly from finer-scale simulations,” according to Fred Streitz, the project’s principal investigator and the LLNL Computing Directorate’s deputy associate director for strategic partnerships. “The model is capable of describing phenomena like lipid-driven protein aggregation and, because of its efficiency, researchers were able to easily explore the possible space of protein arrangements and their lipid environments.”
Development of the framework was part of the NCI/DOE Joint Design of Advanced Computing Solutions for Cancer (JDACS4C) Pilot 2 project focused on developing a greater understanding of RAS-RAF-driven cancer initiation and growth by combining machine learning (ML) and the high-performance computing expertise at the DOE national laboratories with the state-of-the-art experimental capability at NCI to study RAS biology on membranes and advance discovery of new cancer drugs.
Under the pilot project, LLNL scientists demonstrated a Multiscale Machine-Learned Modeling Infrastructure (MuMMI) to simulate RAS protein behavior on a realistic cell membrane, as well as how RAS-RAF proteins interact with each other and with the membrane lipids.
The goal was to deepen understanding of RAS biology through next-generation experimental data, simulations and predictive models. Researchers envision that simulations of RAS and its interactions with the cell membrane will lead to a better biological knowledge and new insights that will accelerate new treatment options for RAS-related cancers.
The follow-on project, ADMIRRAL (AI-Driven Multiscale Investigation of the RAS/RAF Activation Lifecycle), is extending the substantial capability to model RAS biology developed under Pilot 2 to explore a much longer timescale and to address signal-activation pathways.
Machine learning will be used to hypothesize potential configurations along a pathway and then test them, all without human intervention, according to researchers. ADMIRRAL is co-led at LLNL by Streitz and by Cancer Research Technology Program Director Dwight Nissley at the NCI’s Frederick National Laboratory for Cancer Research.
In the future, researchers plan to improve the recently published DDFT model with more accurate anisotropic protein interactions and membrane deformations. Other co-authors included LLNL scientists Tim Carpenter, Helgi Ingolfsson, Mike Surh, Felice Lightstone and Jim Glosli.
More information:
L. G. Stanton et al, Dynamic density functional theory of multicomponent cellular membranes, Physical Review Research (2023). DOI: 10.1103/PhysRevResearch.5.013080
Provided by
Lawrence Livermore National Laboratory
Citation:
Scientists develop model for more efficient simulations of protein interactions linked to cancer (2023, March 28)