A joint research team led by Dr. Akira Kunitomi, a former postdoctoral fellow at CiRA (currently a researcher at the Gladstone Institute of Cardiovascular Disease), and ID Pharma Co., Ltd., has uncovered the crucial role of oocyte-specific linker histone, H1FOO, in enhancing reprogramming efficiency and homogeneity to primed and naïve pluripotent states. The study is published in Stem Cell Reports.
Whereas conventional reprogramming produces human induced pluripotent stem (iPS) cells with “primed” characteristics, resembling post-implantation epiblasts with limited potential to turn into extraembryonic tissues, the “naïve” pluripotent state displays properties more similar to preimplantation epiblast cells and mouse iPS cells, thus allowing them to differentiate into both embryonic and extraembryonic lineages.
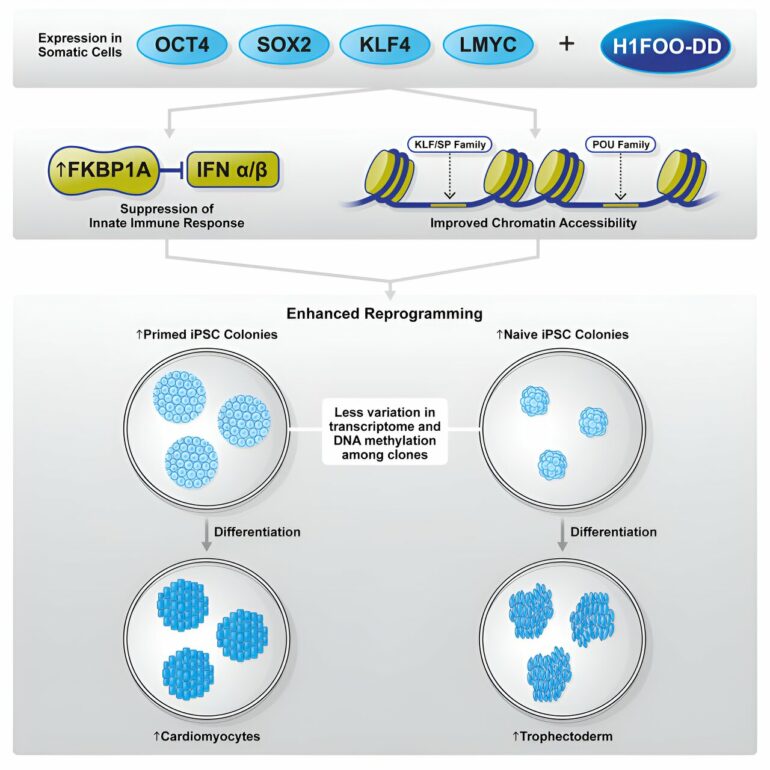
Even though primed and naïve human iPS cells have enabled biomedical advances previously unimaginable, a principal challenge concerning iPS cells for basic research and medical purposes is their heterogeneity. Traditional reprogramming methods remain a stochastic process, and hence, many researchers continue to seek more efficient and precise means to generate homogeneous iPS cells.
In their recent study, the collaborative research team examined the potential of the maternal-specific linker histone H1FOO as a candidate factor to refine the reprogramming process. The researchers engineered H1FOO by attaching a destabilization domain (DD) so they could manipulate its degradation chemically and carefully regulate its levels.
When used in combination with the Yamanaka factors (OSKL, MYC was replaced by LMYC to avoid tumorigenesis), they found H1FOO-DD to improve iPS cell generation efficiency significantly, regardless of the gene delivery system used.
Although gene expression and epigenetic analyses did not identify any meaningful differences between reprogramming using only OSKL or in combination with H1FOO-DD, they did, however, reveal that by using H1FOO-DD, the independently generated iPS cells displayed a more similar gene expression pattern, thus indicating improvements to homogeneity and reproducibility.
Notably, an analysis of genes with highly variable expression between independent iPS cell lines suggested that H1FOO reduced such variability by nearly half. Crucially, many of these genes are involved in gene expression regulation, hence demonstrating the ability of H1FOO-DD to guide the reprogramming process more stringently.
Furthermore, the researchers also observed that iPS cells generated via reprogramming with H1FOO-DD were better at differentiating into endoderm, one of three primary germ layers, and cardiomyocytes (a mesodermal cell type).
The research team continued their investigation by examining the underlying mechanisms through which H1FOO-DD improves reprogramming. By single-cell RNA sequencing (scRNA-seq) analysis, the researchers found reprogramming with H1FOO-DD not only led to earlier and higher expression of pluripotency-related genes but also suppressed the expression of genes related to innate immune response, inflammation, and apoptosis (programmed cell death).
In particular, H1FOO-DD increased the proportion of cells categorized as successfully reprogrammed while reducing cell subpopulations deemed to have undergone incomplete or unsuccessful reprogramming.
Given H1FOO’s role as a chromatin remodeling factor, the researchers also examined accessibility to chromatin regions. Consistent with the findings from gene expression analysis, chromatin regions, especially those nearby pluripotency markers, opened earlier and were more accessible when H1FOO-DD was included as a reprogramming factor.
Further analysis demonstrated that POU and KLF/SP transcription factor families were activated earlier during the reprogramming process by H1FOO-DD. These results suggest that H1FOO-DD helps to coordinate reprogramming more efficiently and timely to enhance iPS cell generation.
The researchers next focused on downstream effectors that help mediate the positive effects of H1FOO-DD on iPS cell generation by examining genes with differential expression early on during reprogramming. Through this analysis, they identified 19 upregulated and two downregulated genes when H1FOO-DD was included as a reprogramming factor.
The research team examined these genes individually to determine whether they influence primed and naïve reprogramming and found that FKBP1A or APOE overexpression improves reprogramming. Since APOE was previously reported as highly expressed during reprogramming, the researchers focused their attention on FKBP1A in hopes of revealing novel molecular mechanisms underlying successful reprogramming.
Notably, they found that while reprogramming using OSKL alone does increase FKBP1A expression, the inclusion of H1FOO-DD dramatically enhances it. This observation was further supported by re-examining the gene expression and chromatin accessibility data they had already collected.
FKBP1A is an immunophilin involved in immunosuppression that can interact with and inhibit TGFBR1, which, in turn, promotes mesenchymal-to-epithelial transition (MET) and improves reprogramming efficiency.
The researchers thus hypothesized that both FKBP1A functions in suppressing innate immunity and inhibiting TGFBR1-mediated MET) are likely responsible for the enhancement of reprogramming efficiency by H1FOO-DD.
Notably, they found that reprogramming via a combination of OSKL and FKBP1A overexpression led to comparable TGFBR1 suppression, MET enhancement, innate immune response suppression, and apoptosis, as when H1FOO-DD was used to initiate reprogramming.
Lastly, because earlier gene expression analysis indicated that in addition to enhancing primed reprogramming, H1FOO-DD also induced the expression of naïve state markers, the researchers examined whether H1FOO-DD is also capable of promoting reprogramming to the naïve state. Indeed, they observed that H1FOO-DD inclusion substantially improved naïve iPS cell generation.
Furthermore, analogous to primed reprogramming, H1FOO-DD reinforced the reprogramming process by coordinating more uniform gene and epigenetic regulation in the cells. Functionally, as measured by metabolic activity and X-chromosome reactivation, reprogramming using H1FOO-DD produced naïve iPS cells more similar to naïve embryonic stem cells or preimplantation blastocysts than when OSKL was used alone.
In summary, combined efforts by the joint research team identified the H1FOO-FKBP1A axis as a means to reprogram cells with greater efficiency and precision. These findings will prove to have a tremendous impact on iPS cell generation for both basic scientific research and clinical applications.
More information:
Akira Kunitomi et al, H1FOO-DD promotes efficiency and uniformity in reprogramming to naive pluripotency, Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2024.04.005
Citation:
Scientists link oocyte-specific histone H1FOO to better iPS cell generation (2024, May 10)