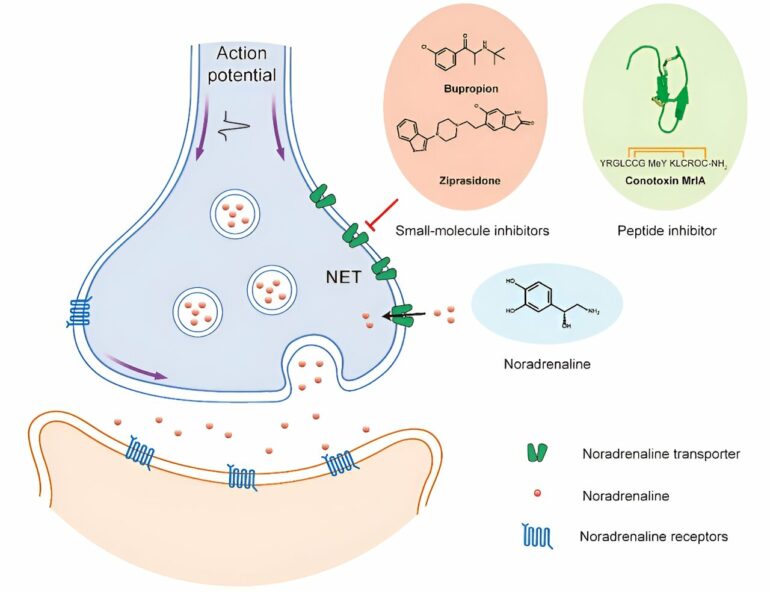
Noradrenaline (NA) is an important monoamine neurotransmitter in the nervous system. The noradrenaline transporter (NET) located on the presynaptic membrane can transport NA into presynaptic neurons.
In clinical applications, various small molecule drugs inhibit NET to increase the concentration of NA in the synaptic cleft, thereby enhancing the signaling of noradrenergic neurons to treat diseases such as depression and attention deficit hyperactivity disorder (ADHD). However, the substrates and binding modes of NET with different drug molecules are still not fully understood.
In a study published in Nature on July 31, Prof. Zhao Yan’s team from the Institute of Biophysics of the Chinese Academy of Sciences, along with collaborators from the University of Copenhagen, Denmark, used single-particle cryo-electron microscopy to resolve the high-resolution structures of human NET and its various complexes.
The research revealed the structural features of NET in multiple functional states and the binding modes of different drug molecules.
The researchers elucidated the structures of inward-open state NETapo and NETNA, both at a resolution of 2.6 Å. The NETNA structure revealed how NET recognizes the substrate NA at its central binding site.
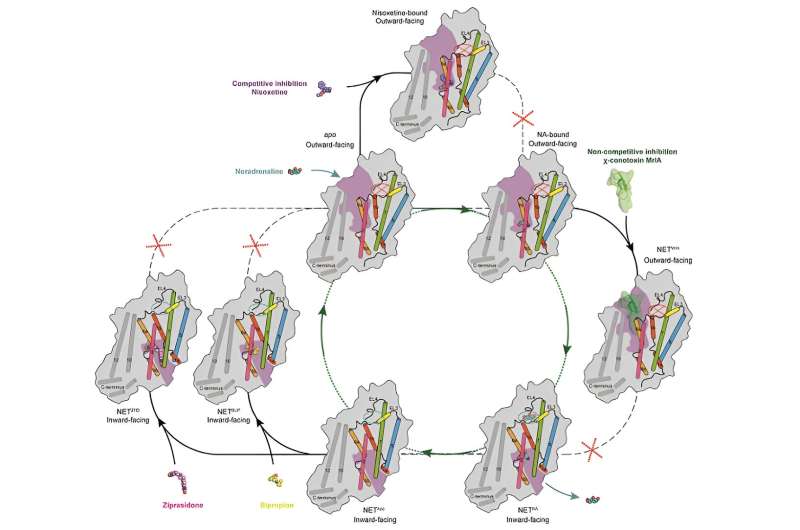
The transport cycle and inhibition modes of NET. © Zhao Yan’s group
They also mapped the binding sites of ions in different transport states of NET, and thus provided a structural basis for understanding the coupling mechanism between ion binding, substrate recognition, and conformational changes.
χ-MrlA is the only known non-competitive inhibitor of NET. Through structural analysis of the complex formed by NET and χ-MrlA, as well as functional experiments with mutants, researchers revealed the crucial structural basis for χ-MrlA’s selective inhibition of NET without affecting other family members such as the serotonin transporter (SERT) and dopamine transporter (DAT).
To uncover the molecular mechanisms by which Bupropion and Ziprasidone, two antidepressants and antipsychotics commonly used in clinical treatment, selectively inhibit the three monoamine transports (MATs)—NET, DAT, and SERT—the researchers analyzed the structures of NET in complex with Bupropion and Ziprasidone.
They found that both Bupropion and Ziprasidone bind in the binding pocket of NET facing the intracellular side.
This study provides a deep understanding of NET’s transport mechanism, offering a crucial structural foundation for the development of novel peptide-based drugs targeting the MATs family. It also provides key insights for developing drugs targeting MATs and offers strong theoretical support for the development of new medications.
More information:
Tuo Hu et al, Transport and inhibition mechanisms of the human noradrenaline transporter, Nature (2024). DOI: 10.1038/s41586-024-07638-z
Provided by
Chinese Academy of Sciences
Citation:
Scientists reveal transport mechanism of norepinephrine transporter and binding mode of small molecule and peptide drugs (2024, August 2)