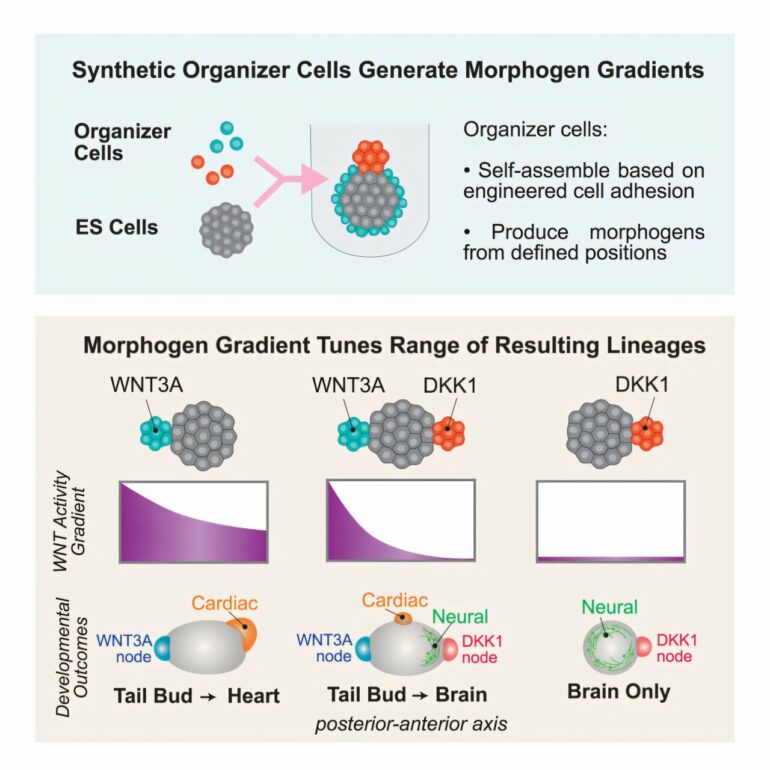
Investigators from Cedars-Sinai and the University of California, San Francisco (UCSF) have identified a new way to deliver instructions that tell stem cells to grow into specific bodily structures, a critical step in eventually regenerating and repairing tissues and organs.
The scientists engineered cells that form structures called “synthetic organizers.” These organizers provided instructions to the stem cells through biochemical signals called morphogens, which stimulated and enabled the stem cells to grow into specific complex tissues and organ-like assemblies.
The research was conducted with mouse embryonic stem cells, and the findings were published in Cell.
“We can use these synthetic organizers to push the stem cells toward making different parts of the early embryo or toward making a heart or other organs,” said Ophir Klein, MD, Ph.D., co-corresponding author of the study, executive vice dean of Children’s Health and executive director of Cedars-Sinai Guerin Children’s.
In one instance, scientists were able to induce the stem cells to begin to form a mouse body that stretched from head to tail, similar to regular embryonic development in the womb. In another instance, the scientists were able to spur the stem cells to generate a large heart-like structure complete with a central chamber and a regular beat, along with a network of early blood vessels.
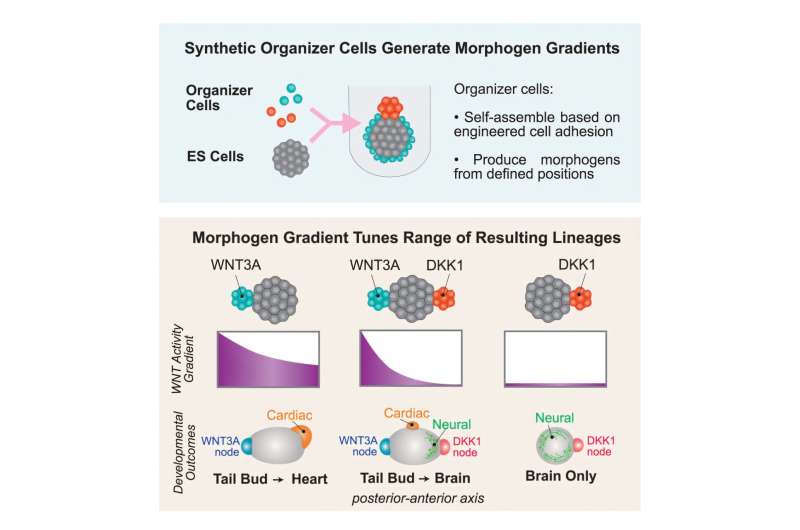
Graphical abstract. © Cell (2024). DOI: 10.1016/j.cell.2024.11.017
“This type of synthetic organizer cell platform provides a new way to interface with stem cells and to program what they develop into,” said Wendell Lim, Ph.D., co-corresponding author and professor of Cellular and Molecular Pharmacology at UCSF.
“By controlling and reshaping how stem cells differentiate and develop, it might allow us to grow better organs for transplantation or organoids for disease modeling and eventually utilize it to drive tissue regeneration in living patients.”
To steer organizer cells and control stem cell development, the scientists uploaded genetic codes into the cells and engineered two key features in the cells.
First, they instructed the cells to stick to the stem cells in the form of a node or a shell clustering around the clump of stem cells. Second, the investigators engineered the organizer cells to produce specific biochemical signals crucial to inducing early embryonic development.
To effectively and precisely control the organizer cells, researchers developed a chemical switch within the cells, allowing scientists to turn the delivery of instructions to stem cells on or off. Additionally, they installed a “suicide” switch to eliminate the organizer cells when needed.
“These synthetic organizers show that we can provide more refined developmental instructions to stem cells by engineering where and when specific morphogen signals are provided,” Lim said. “The organizer cells carry both spatial information and biochemical information, thus giving us an incredible amount of control that we have not had before.”
Klein said the use of engineered synthetic organizer cells could ultimately allow the team to build real-world applications in the future.
“The remarkable science of programming instructions to coax stem cells could one day open the door to tackle complex diseases,” Klein said.
“We could generate specific cell types, like a beta cell to make insulin or a neuron to treat Parkinson’s disease, within the context of a larger piece of tissue or even a whole organ. This work opens many new and exciting possibilities.”
More information:
Toshimichi Yamada et al, Synthetic organizer cells guide development via spatial and biochemical instructions, Cell (2024). DOI: 10.1016/j.cell.2024.11.017
Provided by
Cedars-Sinai Medical Center
Citation:
Scientists steer the development of stem cells to regenerate and repair organs (2024, December 20)