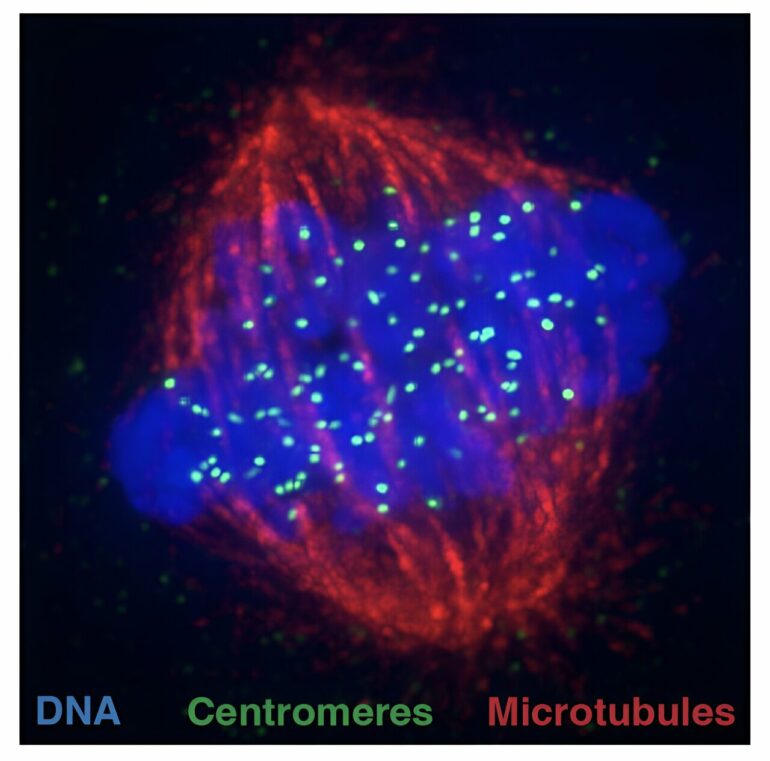
Scientists have solved a decade-long question about the mechanism that preserves the centromere, the hub that ensures DNA divides correctly during cell division.
The study, published in Science, revealed that a protein, known as PLK1, triggers a process that coordinates key proteins at the right place and time during cell division—ensuring each new cell has a centromere in the right location.
The centromere is a region of DNA where the cell division machinery attaches to segregate identical copies of the cell’s genetic material into newly formed cells.
The discovery sheds light on one of life’s most fundamental processes that ensures that the cell’s DNA, packaged into chromosomes, is separated correctly through multiple rounds of cell division.
“In the human body, around two trillion cells divide every day. Accurate chromosome segregation is the basis for life itself and mistakes can be catastrophic. If centromeres are missing or in the wrong place, then the genetic information is not shared correctly between the dividing cells.
“In adults, this can lead to many diseases including cancers, while in the earliest stages of life it can cause birth defects,” says Professor Jeyaprakash Arulanandam, who led this work at the University of Edinburgh and Ludwig-Maximilians-Universität München.
The cell division machinery identifies centromeres by the presence of multiple copies of a protein known as CENP-A. But every time the cell divides, the stocks of this protein at centromeres must be refilled.
Over the years, the precise molecular events that allow this replenishment to occur so that the centromere maintains its identity and location through vast numbers of cell divisions have been the focus of intense research.
Research by another group previously revealed that PLK1 is one of the molecular “master” switches which controls when CENP-A replenishment occurs, but its mechanism of action remained a mystery.
The team, including University of Edinburgh and Ludwig-Maximilians-Universität München researchers, used biophysical, biochemical, structural and cell biology techniques to better understand PLK1 actions.
This study revealed that PLK1 makes a chemical change, known as phosphorylation, to two proteins, known as Mis18α and Mis18BP1, which form part of a set of proteins, known as the Mis-18 complex.
Previous research, including work by Professor Jeyaprakash Arulanandam’s team, had revealed that the Mis18 protein complex plays a vital role in replenishing CENP-A levels as cells divide.
These initial chemical changes create binding sites on the Mis18 complex, allowing the PLK1 protein to make additional phosphorylations to other Mis18 proteins which activates the Mis18 complex.
The researchers found that PLK1 also phosphorylates another protein, known as HJURP, which is responsible for loading CENP-A onto the centromeres.
Together these changes allow the Mis18 complex to act as a guide, controlling when HJURP binds to the centromere and ensuring CENP-A is loaded at the right place and time during cell division.
“PLK1 kickstarts a molecular process similar to a relay race that determines how and when key proteins interact. It ensures that CENP-A levels are restored after each round of cell division, preserving the centromere’s integrity.
“This is one of cell’s most crucial safeguards and is vital to the correct transfer of genetic material through countless generations of cells—which is essential to the creation and maintenance of life,” says Pragya Parashara, one of the lead authors of the paper at the University of Edinburgh.
More information:
Pragya Parashara et al, PLK1-mediated phosphorylation cascade activates Mis18 complex to ensure centromere inheritance, Science (2024). DOI: 10.1126/science.ado8270
Provided by
University of Edinburgh
Citation:
Scientists uncover mechanism preserving centromere during cell division (2024, September 6)