Biophysical chemists from the National University of Singapore (NUS) have uncovered a previously hidden landscape that governs the intracellular organization and dynamics of SWI/SNF chromatin remodelers, an important class of protein complexes that control genome access inside the cell.
The work was published in the journal Nature Communications.
By revealing how such dynamics could go awry in mutant variants of the remodeler proteins linked to diverse human cancers, the insights from this study could potentially serve as a uniquely valuable set of quantitative signatures for these cancer-associated remodeler mutations.
Chromatin remodelers play a key role in regulating access to DNA (which is packed within the cell into chromatin), hence allowing other DNA-interacting processes to take place. Although mutations in the SWI/SNF remodeler subfamily are known to be linked to over 20% of all human cancers, how these mutations disrupt their interactions with DNA to ultimately lead to cancer remains poorly understood in a quantitative manner.
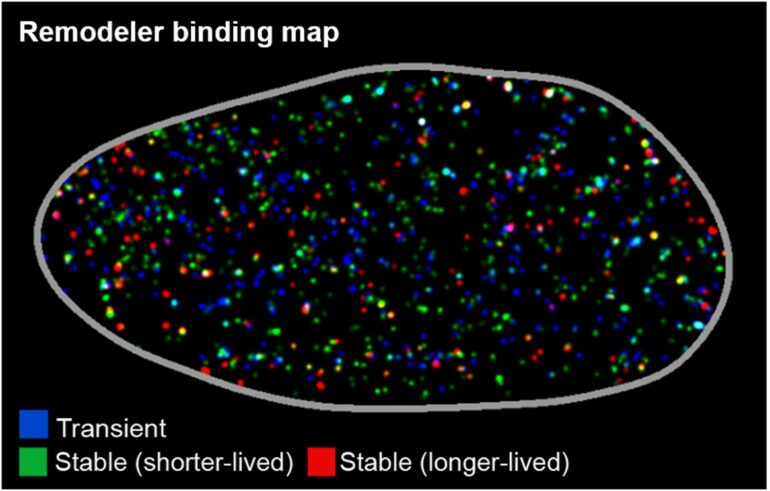
A research team led by Assistant Professor Ziqing Winston Zhao from the Department of Chemistry and the Center for BioImaging Sciences at NUS has used an advanced single-molecule imaging approach to quantify the dynamics of the fully assembled SWI/SNF remodeler complex for the first time in live human cells, resolving distinct modes of intracellular movement and DNA-binding across different timescales.
To better understand the spatial organization of such dynamics, they also devised a super-resolution mapping strategy, called STAR, that enabled them to reveal numerous nanoscale “hotspots” in the cell nucleus where multiple longer-lived DNA-binding events preferentially cluster, ultimately leading to sustained productive chromatin remodeling at these sites.
Finally, by systematically comparing across a variety of remodeler mutants implicated in different cancers across tumor types, the team identified multi-modal changes in DNA-binding dynamics unique to each mutant.
These findings establish the biophysical basis for aberrant remodeler–chromatin interactions in cancer, and could potentially lead to a new set of diagnostic markers for cancer-associated remodeler mutations.
Prof Zhao said, “Our findings provide first-of-its-kind quantitative insights into the organization and dynamics of this critical intracellular process in both space and time, unveiling a much broader regulatory landscape at work than previously thought.
“The approaches developed in our study have also empowered us to visualize, quantify and map a wide range of other DNA-interacting processes, and pave the way to further investigate the in vivo implications of chromatin remodeling misregulation in disease contexts.”
More information:
Wilfried Engl et al, Single-molecule imaging of SWI/SNF chromatin remodelers reveals bromodomain-mediated and cancer-mutants-specific landscape of multi-modal DNA-binding dynamics, Nature Communications (2024). DOI: 10.1038/s41467-024-52040-y
Provided by
National University of Singapore
Citation:
Single-molecule imaging reveals aberrant DNA-binding dynamics of cancer-linked chromatin remodelers (2024, October 18)