Thanks to great technological advances, the genetic material of living beings can now be sequenced at a rapid rate. Comparisons of genomes, whether of closely related or completely different species, reveal particularly interesting findings. In this way, information can be obtained on phylogenetic relationships, the formation of characteristics or on adaptive abilities.
However, comparing genomic data poses tricky technical challenges. To simplify the analysis process, a team of scientists led by Prof. Michael Hiller from the Hessian LOEWE Centre for Translational Biodiversity Genomics (TBG) has developed a new method and presented it in the journal Science.
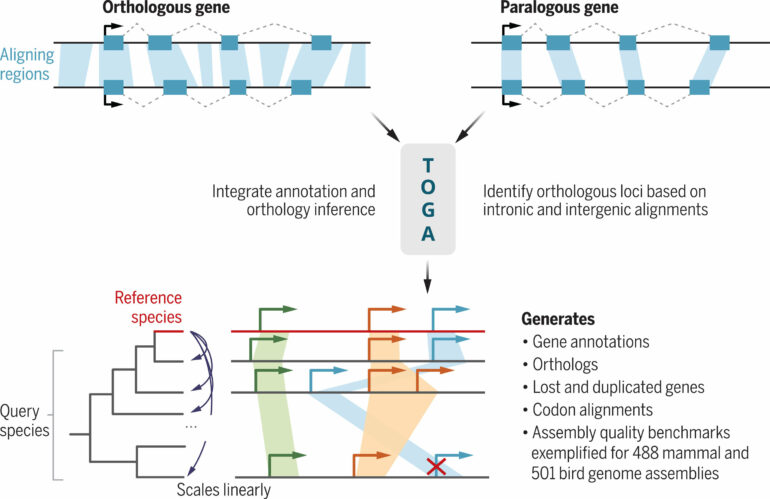
When comparing the genomes of different organisms, scientific knowledge is gained in a two-step process: First, the individual genes within the genome of the respective species must be localized. This process is called gene annotation. Then, for comparison, the second step is to determine which genes in the two organisms correspond to each other; such corresponding genes are called orthologs. Both steps are technically demanding and make it difficult to obtain new information from the genome data to be compared.
The new computational method TOGA simplifies such analyses and tackles both challenges together. The acronym stands for “Tool to infer Orthologs from Genome Alignments.” To determine orthologous genes, researchers use the fact that the parts in the genes that code for proteins are generally more similar to each other than the coding sections of other genes. The TOGA method extends this similarity principle to the entire genomic context of a gene.
“We have near complete genomes, so we might as well utilize them instead of focusing only on the protein-coding parts. By comparing whole genomes of different organisms and using machine learning, we can determine orthologous genes with a very high accuracy,” explains study leader Michael Hiller, Professor of Comparative Genomics at the LOEWE Centre TBG and the Senckenberg Society for Nature Research, who started the project at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden.
The study showed that orthologous genes in other mammalian genomes can be accurately localized just using the known genes from human or mouse. Similarly, known genes from chicken can be used to locate orthologous genes in the genomes of other birds.
“This has allowed us to apply TOGA to the genomes of hundreds of other species. By annotating and determining orthologous genes for more than 500 mammalian and 500 bird genomes, we have generated the largest cross-species gene resources for these vertebrate groups to date. These resources help determine the phylogeny of species and link changes in genes to changes in traits,” Hiller adds.
In addition to Hiller’s team, the study involved scientists from the Zoonomia Consortium, an international consortium of researchers investigating the genomic basis of common and specialized traits in mammals. The aim of the consortium is to use the possibilities of comparative genomics as a tool for human medicine and the conservation of biological diversity.
As part of the Zoonomia Consortium, Hiller and other Senckenberg researchers are also involved in the study “Evolutionary constraint and innovation across hundreds of placental mammals,” which is published in the same issue of Science and investigates the evolution of mammalian genomes.
More information:
Bogdan M. Kirilenko et al, Integrating gene annotation with orthology inference at scale, Science (2023). DOI: 10.1126/science.abn3107
Provided by
Senckenberg Research Institute and Natural History Museum
Citation:
Sophisticated gene memory: Researchers develop new method to genetically compare hundreds of animal species (2023, April 27)