Apomixis is a form of asexual reproduction that allows hybrid rice to propagate by seeds. Recently, a collaborative research team led by Professor Li Jiayang from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Science established a new synthetic apomixis system using the rice endogenous gene OsWUS, which enables normal seed-setting rates.
Results of this study are published in Plant Communications.
The cultivation and widespread use of hybrid rice have played a crucial role in enhancing grain yields and ensuring food security in China. However, due to genetic recombination and trait segregation, hybrid rice cannot be used for seed saving like conventional rice. This necessitates labor-intensive and costly hybrid seed production efforts by breeders and seed companies every year, and farmers must repurchase new seeds annually.
In previous synthetic apomixis systems, the genes responsible for inducing parthenogenesis were initially discovered in plants capable of natural apomixis. In this study, researchers found that the rice endogenous gene OsWUS could be regulated to induce apomixis and achieve clonal seed production without affecting seed-setting rates.
OsWUS, also known as MOC3, plays a vital role in regulating the formation and development of tiller buds, as demonstrated in earlier studies by Li’s team. Mutations in MOC3 result in impaired tiller bud formation and significantly reduced tiller numbers.
Moreover, overexpression of WUS can promote somatic embryogenesis and shoot regeneration in plants like Arabidopsis.
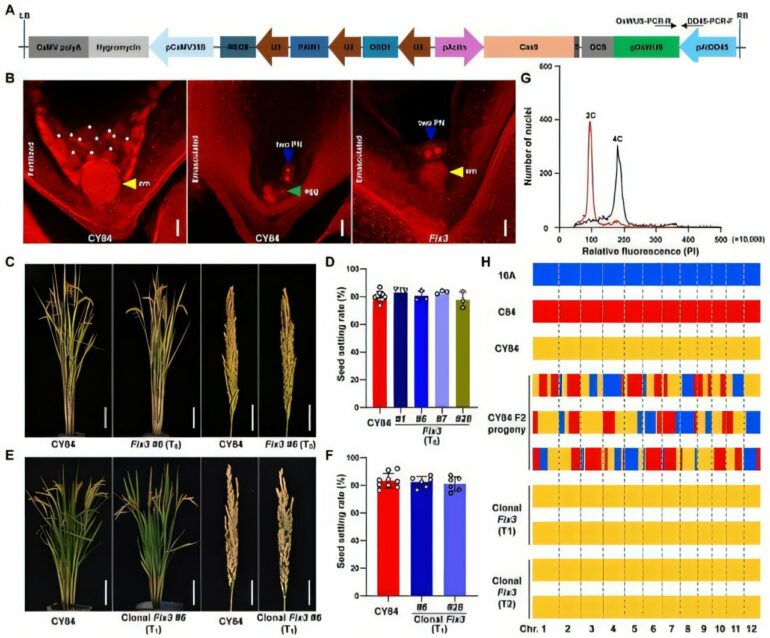
Collaborating with Yazhouwan National Laboratory and China National Rice Research Institute, Li’s team constructed an ectopic expression vector for OsWUS, driven by the Arabidopsis egg cell-specific promoter pAtDD45, and transformed it into hybrid rice Chunyou 84 (CY84).
During the transformation of OsWUS alone, challenges such as difficulties in plant regeneration, dwarfism, and sterility were observed. However, by combining ectopic expression of OsWUS with the MiMe strategy (Mitosis instead of Meiosis), the researchers obtained Fix3 (Fixation of hybrids 3) lines that showed normal growth and development.
Confocal microscopy revealed that approximately 20% of emasculated Fix3 ovules exhibited embryo development independent of fertilization, indicating that egg cell-specific expression of OsWUS could partially replace the fertilization process and induce parthenogenesis.
In addition, the Fix3 lines exhibited agronomic traits similar to those of wild-type hybrid rice, including completely normal seed-setting rates and a high clonal seed efficiency, with some lines reaching a cloning efficiency of approximately 21.7%.
Further phenotypic analysis indicated that the progeny of these clonal plants showed phenotypic characteristics highly similar to those of the wild-type hybrid rice and maintained normal seed-setting rates.
The Fix3 apomixis system based on OsWUS also demonstrated varying levels of clonal seed efficiency across different lines, significantly surpassing the Fix2 system established using OsBBM4. This indicates a greater potential for practical application.
However, compared with apomixis systems based on OsBBM1 and dandelion PAR genes, the cloning efficiency of the current OsWUS-based system still needs to be improved.
In the future, mining additional egg cell-specific promoters or incorporating more endogenous genes that do not affect seed-setting rates may further enhance cloning efficiency while maintaining normal seed yields.
Additionally, since homologs of OsWUS exist in multiple crops, this system could potentially be extended to other hybrid crops, facilitating broader utilization of hybrid vigor.
More information:
Yong Huang et al, OsWUS-driven synthetic apomixis in hybrid rice, Plant Communications (2024). DOI: 10.1016/j.xplc.2024.101136
Provided by
Chinese Academy of Sciences
Citation:
Synthetic asexual reproduction system in hybrid rice shows promise for seed production (2024, October 24)