by Julia Reichelt, Rheinland-Pfälzische Technische Universität Kaiserslautern-Landau
Viruses that infect bacteria—known as bacteriophages—could be used in a targeted manner to combat bacterial diseases. They also play an important ecological role in global biogeochemical cycles. Recent research by researchers at the University of Kaiserslautern-Landau (RPTU) has identified a previously unknown auxiliary metabolic gene in aquatic phages, thereby significantly expanding the previous understanding of these bacterial predators.
The work is published in the journal Nature Communications.
Phages are viruses that exclusively attack bacteria. The goal of numerous scientists is to learn more about these tiny replicative units, measuring between 20 and 300 nm (a hair is 80,000 nm thick).
“If we understand the details of how phages ultimately infect and kill bacteria, then in the future we might be able to use them specifically against harmful bacteria,” explains Professor Nicole Frankenberg-Dinkel from the RPTU. The microbiology team is investigating the various strategies phages use to turn bacteria into “factories” for their replication, that is, the production of hundreds of new phages.
“We are particularly interested in aquatic habitats, particularly oceans and lakes, because phages occur in high numbers, and they play an important ecological role in nutrient recycling,” says Prof. Frankenberg-Dinkel.
The long-term goal of the bacteriophage research field is not only to apply phage therapy to combat “bad” bacteria responsible for diseases, but also—sticking with aquatic habitats—to address the ecological role of phages in the global nutrient cycles.
Phages play a crucial ecological role in aquatic environments by controlling bacterial populations, maintaining microbial diversity, and influencing nutrient cycling through processes like the viral shunt. They also drive microbial evolution by promoting horizontal gene transfer—transmission from one organism to another, rather than–as is typically the case, from generation to generation—and exerting selective pressure on bacteria.
In a recent study, Frankenberg-Dinkel’s team—in collaboration with researchers from Israel, the Netherlands, Tübingen and Stechlin/Potsdam—analyzed phage genetic material from environmental samples using bioinformatics.
“Normally, this genetic material mainly contains the blueprint information for producing new phage particles. The phages then use the bacteria as factories,” says the professor.
However, the researchers also found “auxiliary metabolic genes” in the phage genetic material. These auxiliary genes originally come from bacteria and were once hijacked by the phages. They are not required for the assembly of new phage particles, but instead serve to “reprogram” the host—namely, the bacteria—during a phage infection.
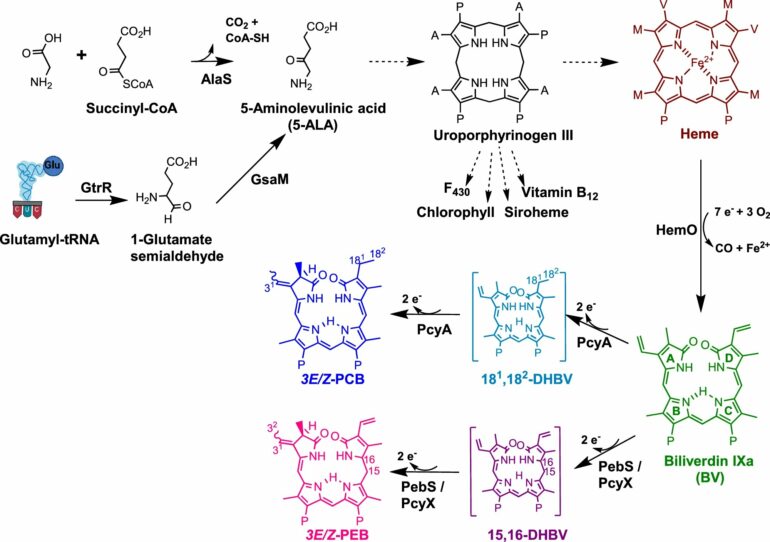
“In our study, we have discovered a previously unknown auxiliary metabolic gene in the phages,” says Frankenberg-Dinkel, explaining her latest results. “We were able to show that this gene codes for an active protein that is important for the biosynthesis of the ‘pigments of life.'”
Tetrapyrroles are referred to as the pigments of life. The most important representatives of these chemical compounds are heme, a component of hemoglobin in blood for oxygen transport, and chlorophyll, the green leaf pigment essential for photosynthesis.
Frankenberg-Dinkel notes, “Our results suggest that tetrapyrroles play an important role during a phage infection. They seem to be so important that phages carry this additional genetic material because it is somehow beneficial to them.”
“The importance of tetrapyrroles for phage infection was not previously known to this extent. Tetrapyrroles are essential for energy production in cells,” Frankenberg-Dinkel continues. “We suspect that there is an increased energy demand when bacteria have to produce phage particles. Therefore, more tetrapyrroles may be needed.”
The researchers were able to demonstrate that the auxiliary metabolic gene is present in phages identified in both salt and fresh water.
According to Frankenberg-Dinkel, the current study results reveal another interesting finding: there are two ways to produce the first precursor of tetrapyrroles, one of which is the so-called Shemin pathway. And it is precisely this pathway—or rather the genetic makeup required for it—that the researchers have identified in the phages.
“The Shemin pathway is only found in one group of bacteria, and otherwise only in birds and mammals. This means that the phages must have acquired this gene from a particular group of bacteria. Perhaps because the Shemin pathway is more efficient than the alternative C5 pathway, as it only requires one enzyme instead of two,” she concludes.
More information:
Helen Wegner et al, Identification of Shemin pathway genes for tetrapyrrole biosynthesis in bacteriophage sequences from aquatic environments, Nature Communications (2024). DOI: 10.1038/s41467-024-52726-3
Provided by
Rheinland-Pfälzische Technische Universität Kaiserslautern-Landau
Citation:
Targeting bacteria: Auxiliary metabolic genes expand understanding of phages and their reprogramming strategy (2024, October 15)