Effective drugs against viral diseases like COVID-19 are urgently needed now and in the future. The emergence of viral mutants and yet unknown viruses could push vaccines to their limits.
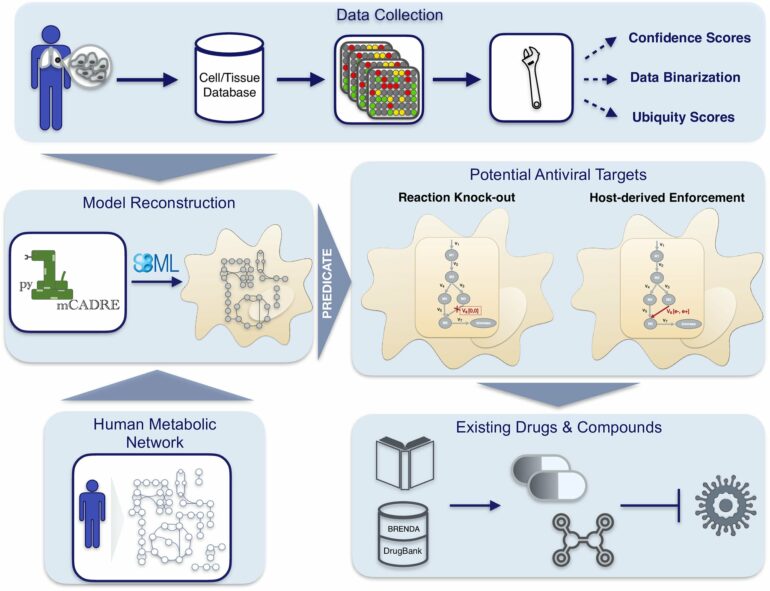
DZIF scientist and bioinformatician Andreas Dräger from the University of Tübingen is working on a computer-based method that can help to accelerate the time-consuming identification and development of antiviral agents. Using a novel analysis technique that applies to any virus and host cell type, the research team around Dräger has now created a model to detect additional host cell targets that allow inhibiting SARS-CoV-2 replication.
“Efficient pandemic preparedness requires new, broadly effective antiviral drugs against which the viruses cannot quickly develop resistance,” explains Dräger, junior professor at the University of Tübingen and member of the Tübingen Cluster of Excellence Controlling Microbes to Fight Infections (CMFI). “But drug development takes too much precious time, which is urgently needed in an emergency.”
Dräger wants to remedy this situation through computer modeling. In 2021, the Tübingen research group identified a human enzyme in the model—guanylate kinase 1—which is indispensable for virus replication and can be switched off without damaging the cell. Now, the bioinformatician has developed a new model with his colleagues to examine the effectiveness of their targets. “Through an improved analysis technique, we can now specifically model viral infection in many different types of tissue,” explains Nantia Leonidou, first author of the current study.
Observing host metabolism after viral infection in the model
Using their integrated systems biology model to simulate infection with SARS-CoV-2 in bronchial epithelial cells, the researchers can identify host-based metabolic pathways that can be inhibited to suppress viral replication.
“If you know the composition of a virus, you can run different scenarios and see how the biochemical reactions in host cells change during viral infection,” Dräger says. The team developed high-quality software to simulate an infection in a cell-type-specific manner.
New targets identified
Applying the model to another cell type, the research group confirmed the previously identified target, guanylate kinase 1, and discovered other new biochemical targets with remarkable antiviral effects. The most promising new hit was CTP synthase 1. Inhibition of this enzyme in the model reduced viral growth by 62 percent without affecting the maintenance of the human host cells.
Both target molecules are closely linked to the structure of genetic material, which requires the same building blocks in both the virus and the host cells. Dräger’s team believes these findings provide a crucial basis for accelerating the development of viral inhibitors.
“Our models could represent a paradigm shift in drug development and accelerate the preclinical phase,” emphasizes Nantia Leonidou, adding, “The methods are fully transferable to any virus and host cell type and are also commercially viable.”
The study is published in PLOS Computational Biology. Dräger’s group now plans to apply their methods to other viruses. The first inhibitors for their discovered enzymes will be tested in animal models for safety, toxicity, and efficacy.
More information:
Nantia Leonidou et al, New workflow predicts drug targets against SARS-CoV-2 via metabolic changes in infected cells, PLOS Computational Biology (2023). DOI: 10.1371/journal.pcbi.1010903
Provided by
Universitaet Tübingen
Citation:
Using targeted computer modeling to accelerate antiviral drug development (2023, March 23)