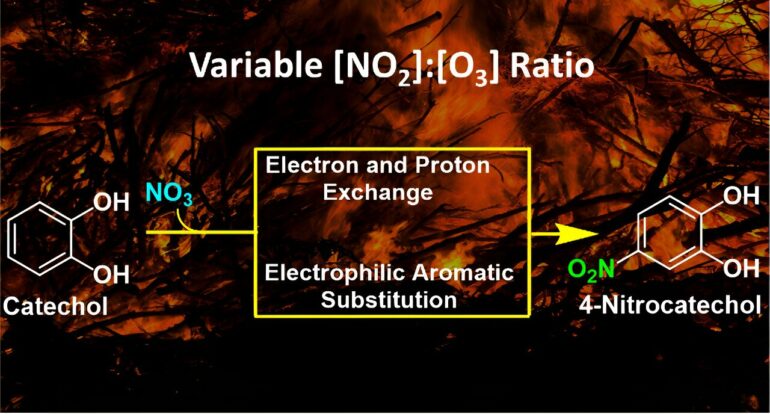
Biomass burning from wildfires puts large amounts of aromatic hydrocarbons in the atmosphere every year, which are thought to convert into more light-absorbing and toxic nitroaromatics.
“This is a really interesting problem and not easy to investigate,” said Marcelo Guzman, a professor of chemistry at the University of Kentucky, explaining his research group publication, “Conversion of Catechol to 4-Nitrocatechol in Aqueous Microdroplets Exposed to O3 and NO2,” in the journal ACS ES&T Air.
It was an afternoon in late July, and Guzman was concerned because the air quality in central Kentucky had reached an unhealthy level due to the wildfire smoke drifting south from Canada. Thick haze from wildfire smoke can also make difficult the vision of pilots from the cockpit of jets and affect the transportation industry.
In the laboratory, a group of enthusiastic students were analyzing chemicals in calibrated instruments to understand how air pollutants interact with the surface of particulates. “Fifteen seconds, then take a sample,” Guzman recommended to the student. The laboratory was bright and relatively quiet, and he wanted to help the student register observations soon after mixing the chemicals.
This was the first visit to Guzman’s laboratory studying environmental chemistry as part of an ambitious National Science Foundation project investigating how pollutants in air are transformed on the surface of particulates suspended in the atmosphere.
The project is identifying new reaction pathways followed by catechols, a group of abundant molecules emitted in the smoke from biomass burns and combustion and determine, among other things, how and when the end products from their transformations absorb more sunlight and also affect public health.
Never before has the catechol molecule been studied in such detail to react so quickly with variable concentration ratios of two key atmospheric molecules, ozone and nitrogen dioxide, representing different scenarios from relatively clean air to very polluted smoke situations.
Although catechols are among the most abundant aromatic hydrocarbons emitted to air during biomass burns, “there are very few studies that have distinguished their specific transformations when in contact with two abundant gases in smoke, nitrogen dioxide and ozone, which create the very reactive nitrate radical, and can imprint a brownish color to the particles that also makes them more toxic,” said Guzman.
We know that the incorporation of a nitro group to the aromatic hydrocarbon ring increases the toxicity of pollutants as occurs on the surface of fine particulate matter in the smoke based on this research.
The World Health Organization reported in 2019 that exposure to ambient (outdoors) air pollution, in both cities and rural areas, causes 4.2 million premature deaths worldwide per year; this mortality is due to exposure to fine particulate matter, which causes cardiovascular and respiratory disease, and cancers. What we did not understand before was how and under what conditions these toxic components form in biomass smoke.
By careful laboratory simulations of reactions under the variable concentrations of chemicals in the smoke the researchers from the University of Kentucky hope to improve the fundamental understanding of transformations in wildfire emissions, which in turn can inform air quality and climate models.
To demonstrate how his lab findings apply to the real world, Guzman opened a file on his laptop to show how the measured production of the nitroaromatic product increased relative to the formation of more nitrate radical. He found that even for the smallest concentration of nitrate radical that should exist at night in the proximity of biomass smoke, the process is highly efficient. Before this work, no one had thought to look at such a dependence.
“We used a special reactor that samples so quickly the interface of water microdroplets without interference of other heterogeneous reactions provided by the wall of the container,” he says. “The atmospheric science community was not considering these unknown reactions in smoke that imply the transfer of electrons and protons between the chemicals as well as the fact that a radical attack can proceed on the surface of water.”
“In our experiments, as soon as the microdroplets containing catechol (the aromatic marker of pollution in smoke) are created, they are stricken by a flow of gas with the very short-lived nitrate radical,” said Guzman. “The interaction of these molecules on the surface of water particles lasts less than a millionth of a second, a time that is long enough to create nitrocatechol, a brown carbon product, which is quickly and unambiguously detected by a state-of-the-art mass spectrometer.”

The Donnie Creek wildfire in 2023, British Columbia, Canada. © BC Wildfire Service
By determining the mass-to-charge ratio of the molecules formed, the researchers can resolve how pollution in smoke becomes worse hundreds of miles away from the emission point. In essence, the environmental conditions near a smoke plume during nighttime are such that they can augment the production of the toxic brown carbon products and PM2.5 from catechol. Moreover, this catechol chemistry contributes to explaining why the mixing of smoke with urban pollution creates significant amounts of nitroaromatics with high yield at low altitude results in a considerable air quality drop.
While the Clean Air Act in 1970 helped to greatly improve the air quality in U.S. cities, when biomass burning from wildfires reaches urban areas, smoke deteriorates those gains and warnings are issued to protect people’s health with recommendations to remain indoors. The American Lung Association reports that in 2023 eight out of the ten most polluted cities in the U.S. by measure of ozone are in the West.
By measure of year-round particulate matter with a diameter of 2.5 microns or smaller (PM2.5) that can penetrate deep into the human lungs during inhalation and later enter the bloodstream, the top 25 cities include Midwest and Southern centers such Indianapolis, Louisville, Houston, Laredo, Birmingham, Cincinnati, and Chicago. Concerns are rising that such trends could worsen as researchers from the United States Forest Service projected that fire emissions should increase by ~50% from 2001–2010 to 2050–2059.
Importantly and directly connected to this problem is the changing climate, with higher temperatures registered by the U.S. National Oceanic and Atmospheric Administration (NOAA), it appears that anthropogenic warming is to blame. Just in the last 50 years, the contiguous 48 states have experienced an average increase of 1.2ºC. The same observation of a 1.2ºC increase can be made for Canada’s annual average temperature but keeping in mind that it becomes more pronounced toward the north of 60 degrees latitude.
Thus, these changes are causing the West and North of the American continent to become not only warmer but also drier, increasing the chance of wildfire occurrence. Considering that 2.2% of the entire globe burned in the year 2022, wildfires and their pollution are not an exclusive problem of the United States.
More information:
Md Sohel Rana et al, Conversion of Catechol to 4-Nitrocatechol in Aqueous Microdroplets Exposed to O3 and NO2ACS ES&T Air (2023) DOI: 10.1021/acsestair.3c00001. pubs.acs.org/doi/10.1021/acsestair.3c00001
Provided by
University of Kentucky
Citation:
How are toxic brown carbon nitroaromatics produced in biomass smoke? (2023, December 5)