As the U.S. Environmental Protection Agency cracks down on insidious “forever chemical” pollution in the environment, military and commercial aviation officials are seeking ways to clean up such pollution from decades of use of fire suppressant foams at military air bases and commercial airports.
Fire-suppression foams contain hundreds unhealthful forever chemicals, known by chemists as PFAS or poly- and per-fluoroalkyl substances. These compounds have stubbornly strong fluorine-to-carbon bonds, which allow them to persist indefinitely in the environment, hence the moniker “forever chemicals.” Also found many other products, PFAS compounds now contaminate groundwater supplies tapped by municipal water suppliers at many locations throughout the nation.
Because they are linked to higher risks for certain cancers and other maladies, the EPA imposed a new rule last month requiring water utilities to reduce contamination if levels exceeded 4 parts per trillion for certain PFAS compounds.
Fortunately, a collaborative discovery by scientists at UC Riverside (UCR) and Clarkson University in Potsdam, N.Y., provides a new strategy to clean up these pollutants.
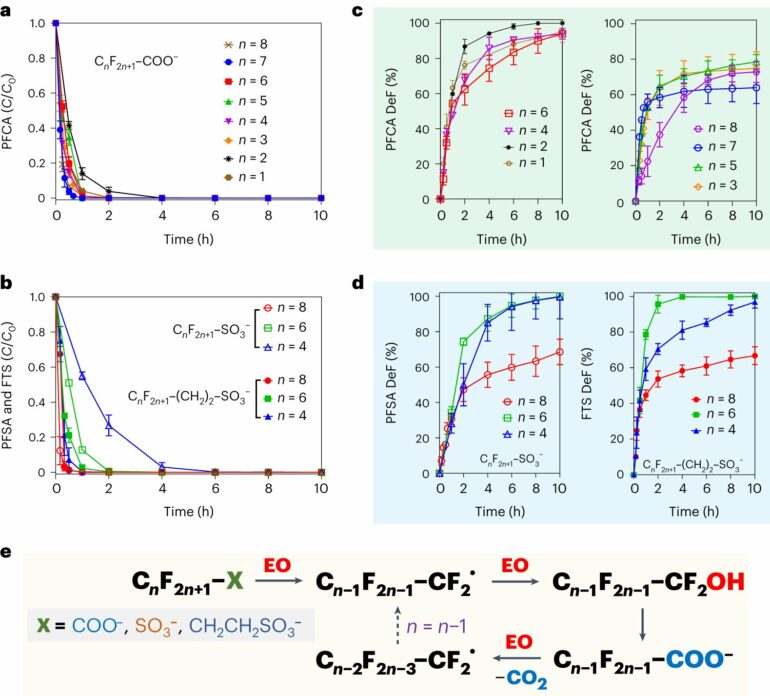
The method is detailed in the journal Nature Water. It involves treating heavily contaminated water with ultra-violet (UV) light, sulfite, and a process called electrochemical oxidation, explained UCR associate professor Jinyong Liu.
“In this work, we continued our research on the UV-based treatment, but this time, we had a collaboration with an electrochemical oxidation expert at Clarkson University,” said Liu, who has published nearly 20 papers on treating PFAS pollutants in contaminated water. “We put these two steps together and we achieved near-complete destruction of PFAS in various water samples contaminated by the foams.”
Liu said the collaboration with a team led by assistant professor Yang Yang at Clarkson solved major technical problems. For instance, the foams contain various other concentrated organic compounds that hinder the breakup of the strong fluorine-to-carbon bonds in the PFAS compounds.
Liu and Yang, however, found that electrochemical oxidation also breaks up these organics. Their process also allows these reactions to occur at room temperature without a need for additional heat or high pressure to stimulate the reaction.
“In the real world, the contaminated water can be very complicated,” Liu said. “It contains a lot of things that might potentially slow down the reaction.”
PFAS compounds have been used in thousands of products ranging from potato chip bags to non-stick cookware, but fire-suppressing foams are a major source of PFAS pollution in groundwater because have been used for for decades to extinguish aviation fuel fires at hundreds of military sites and commercial airports. These foams were also routinely applied to minor fuel spills as a precautionary measure to prevent fires.
Invented by the U.S. Navy in the 1960s, the foams form an aqueous film around burning gasoline and other flammable liquids, which quickly deprives the fire of oxygen and extinguishes it.
Because of widespread use, the Department of Defense ordered assessments of 715 military sites nationally for PFAS releases, and by the end of last year, found that 574 of these sites need further investigations or cleanups as required by federal law.
PFAS cleanups became more urgent last month when the EPA imposed a new rule requiring water utilities to reduce contamination if levels exceeded 4 parts per trillion for certain PFAS compounds.
Liu said the method he developed with Yang is well suited for cleansing heavily contaminated water used to flush out tanks, hoses, and other firefighting equipment. The method also can be used to treat leftover containers of PFAS-containing foams.
Their method can also help water utilities deal with groundwater pollution. Contaminated groundwater is often treated through ion exchange technologies in which the PFAS molecules glob onto resin beads in large treatment tanks. The UV light and electrochemical oxidation method developed by Liu and Yang also can assist the regeneration of beads so they can be recycled, Liu said.
“We want to have sustainable management of the resin,” Liu said. “We want to reuse it.”
The study’s title is “Near-complete destruction of PFAS in aqueous film-forming foam by integrated photo-electrochemical processes.” In addition to Liu and Yang, its authors are Yunqiao Guan, Zekun Liu, Nanyang Yang, Shasha Yang, and Luz Estefanny Quispe-Cardenas, who are current or former graduate students at UCR and Clarkson.
More information:
Yunqiao Guan et al, Near-complete destruction of PFAS in aqueous film-forming foam by integrated photo-electrochemical processes, Nature Water (2024). DOI: 10.1038/s44221-024-00232-7
Provided by
University of California – Riverside
Citation:
New ‘forever chemical’ cleanup strategy discovered (2024, May 9)