Rivers, lakes and oceans worldwide are home to trillions of pieces of plastic pollution that are smaller than five millimeters in length, known as microplastics, and their size allows them to easily enter humans and animals. Some can adsorb and transport other harmful toxicants that pollute waterways, including certain types of a more recently discovered set of toxic “forever chemicals” called per- and polyfluoroalkyl substances, or PFAS.
There are thousands of types of PFAS in freshwater and saltwater bodies, and testing whether microplastics can absorb each one requires costly, time-consuming and labor-intensive testing. That’s why a University of Maine-led team of researchers developed a new type of model for predicting whether any given kind of microplastic would adsorb any specific type of PFAS and at what concentration.
Dilara Hatinoglu, a Ph.D. student in civil and environmental engineering, spearheaded the project in collaboration with Onur Apul, assistant professor of environmental engineering and her adviser, and François Perreault, associate professor at the Arizona State University School of Sustainable Engineering and the Built Environment.
Dilara Hatinoglu. © University of Maine
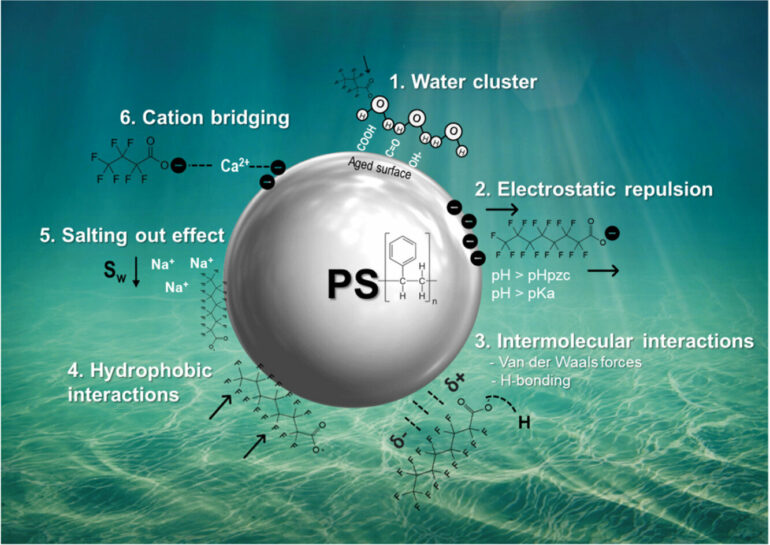
Their models are applicable for fresh and saltwater and account for the type, size, shape and ionic charge of the microplastics; the functional compound groups and chain length of PFAS; and the salinity, pH level and natural organic matter that make up the solution chemistry of the water. In addition to minimizing the need for extensive lab testing, these models could assist with the development of new technologies and resources for PFAS removal.
“In water treatment plants, we use polymers that act as adsorbents, and they have the potential for removing PFAS from water. Our model can support the development of new adsorbent technologies,” says Hatinoglu. “We can modify sorbents depending on the outcomes and findings of our model because it gives an idea of what are the mechanisms and contributors of the adsorption mechanisms.”
For their research, the UMaine-led team trained its models to determine whether polystyrene microplastics would absorb 12 different chemicals, all of which are part of a subset of PFAS known as perfluoroalkyl carboxylic acids (PFCAs). In addition to validating the efficacy of their models, which accounted for both fresh and saltwater, they also obtained several insights into the adsorption mechanisms between PFAS and polystyrene microplastics.
Among their findings were that polystyrene adsorbed greater concentrations of long chain PFAS than short chain ones, microplastics in saltwater adsorb more PFAS than those in freshwater, and the top contributing factors for whether a PFCA will be absorbed into a microplastic is the polarizability and hydrophobicity of the former. Their findings and other details about the model were published in the journal Science of The Total Environment.
Researchers created their method for forecasting PFAS adsorption by microplastics through reconfiguring an existing type of model known as a linear solvation energy relationships (LSER)-based predictive model. Traditional LSER models are used for the adsorption mechanics between naturally-charged organic compounds, but PFAS are negatively-charged. Hatinoglu, Apul and Perreault are among the first researchers to apply LSER modeling to PFAS adsorption.
Hatinoglu is building on her previous work by creating another model to predict the adsorbability of other pollutants into microplastics. The new model will factor in the extent of microplastic degradation.
“The interactions can be significantly affected if the plastics are degraded, so I would like to know more about the effect of degradation on adsorption,” she says. “That would be more realistic.”
The project led by Hatinoglu is part of a broader effort involving Apul, his students and colleagues, and researchers from Arizona State University to explore the interactions between microplastics and various chemicals.
“We are trying to pioneer academic knowledge. We are trying to lead the world and line up with the needs of the state. It’s just a good position to be at,” says Apul. “We’re making [cutting-edge] research in this field, trying to publish the first-evers of this discipline that happens to be at the forefront of Maine and national needs.”
Many UMaine researchers are working together on several PFAS research projects as part of the UMaine PFAS+ Initiative. Apul is science lead and a steering committee member for the university-wide initiative to focus on the emerging PFAS pollution crisis and its cascading environmental and societal impacts.
“We are trying to create a multidisciplinary, collaborative environment to tackle the PFAS problem. It’s such a massive crisis, that a single discipline, a single person is not going to solve it,” Apul says. “There are so many different aspects, so we are trying to bring a multitude of talented researchers. Dilara is one of them, and we’re lucky to have her.”
More information:
M. Dilara Hatinoglu et al, Modified linear solvation energy relationships for adsorption of perfluorocarboxylic acids by polystyrene microplastics, Science of The Total Environment (2022). DOI: 10.1016/j.scitotenv.2022.160524
Provided by
University of Maine
Citation:
New model for predicting adsorption of PFAS by microplastics (2023, May 15)