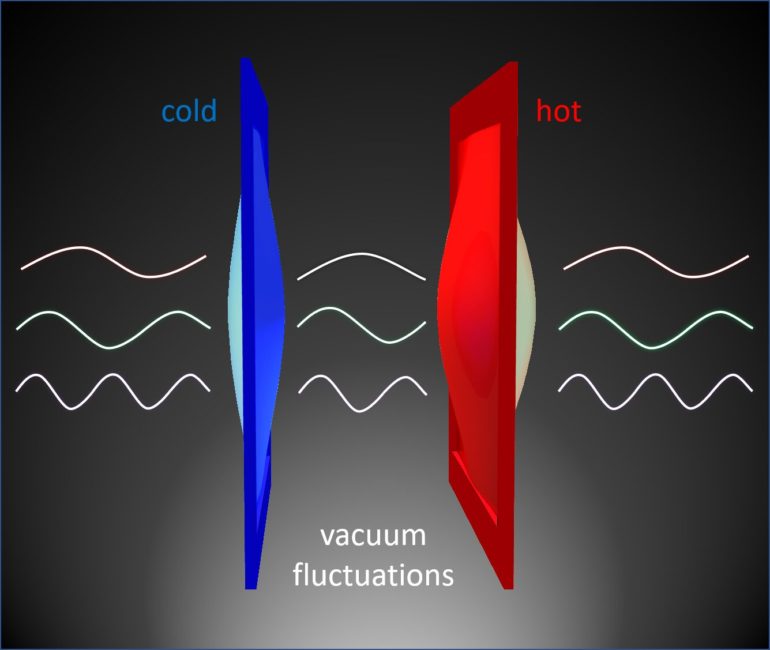
If you use a vacuum-insulated thermos to help keep your coffee hot, you may know it’s a good insulator because heat energy has a hard time moving through empty space. Vibrations of atoms or molecules, which carry thermal energy, simply can’t travel if there are no atoms or molecules around.
But a new study by researchers at the University of California, Berkeley, shows how the weirdness of quantum mechanics can turn even this basic tenet of classical physics on its head.
The study, appearing this week in the journal Nature, shows that heat energy can leap across a few hundred nanometers of a complete vacuum, thanks to a quantum mechanical phenomenon called the Casimir interaction.
Though this interaction is only significant on very short length scales, it could have profound implications for the design of computer chips and other nanoscale electronic components where heat dissipation is key. It also upends what many of us learned about heat transfer in high school physics.
“Heat is usually conducted in a solid through the vibrations of atoms or molecules, or so-called phonons—but in a vacuum, there is no physical medium. So, for many years, textbooks told us that phonons cannot travel through a vacuum,” said Xiang Zhang, the professor of mechanical engineering at UC Berkeley who guided the study. “What we discovered, surprisingly, is that phonons can indeed be transferred across a vacuum by invisible quantum fluctuations.”