The cells in your body are not all the same. Each of your organs has cells with very different functions. For example, liver cells are top-notch secretors, as their job requires them to make and export many of the proteins in your blood. By contrast, muscle cells are tasked with facilitating the contractions that allow you to move.
The fact that cells are so specialized has implications for gene therapy, a way to treat genetic diseases by correcting the source of the error in a patient’s DNA. Health providers use a harmless viral or bacterial vector to carry a corrective gene into a patient’s cells, where the gene then directs the cell to produce the proteins necessary to treat the disease. Muscle cells are a common target because gene therapies injected into the muscle are more accessible than introduction into the body by other routes. But muscle cells may not produce the desired protein as efficiently as needed if the job the gene instructs it to do is very different from the one it specializes in.
We are cell biologists and biophysicists who study how healthy proteins are produced and maintained in cells. This field is called protein homeostasis, also known as proteostasis. Our recently published study details a way to make muscle cells behave more like liver cells by changing protein regulation networks, enhancing their ability to respond to gene therapy and treat genetic diseases.
Gene therapy involves replacing a defective gene with a functioning one that can direct cells to produce missing or dysfunctional proteins.
Boosting protein factories
One disease for which gene therapy has great potential is alpha-1 antitrypsin (AAT) deficiency, a condition in which liver cells are unable to make adequate amounts of the protein AAT. It results in a breakdown of lung tissue that can cause serious respiratory problems, including the development of severe lung diseases such as chronic obstructive pulmonary disease (COPD) or emphysema.
Patients are usually treated by receiving AAT via infusion. But this requires patients to either make regular trips to the hospital or keep expensive equipment at home for the rest of their lives. Replacing the faulty gene that caused their AAT shortage in the first place could be a boon for patients. Current gene therapies inject the AAT-producing gene into muscle. One of our colleagues, Terence Flotte, developed a way to use a harmless version of an adeno-associated virus as a vehicle to deliver AAT gene therapies into the body via injection, allowing for sustained release of the protein over several years.
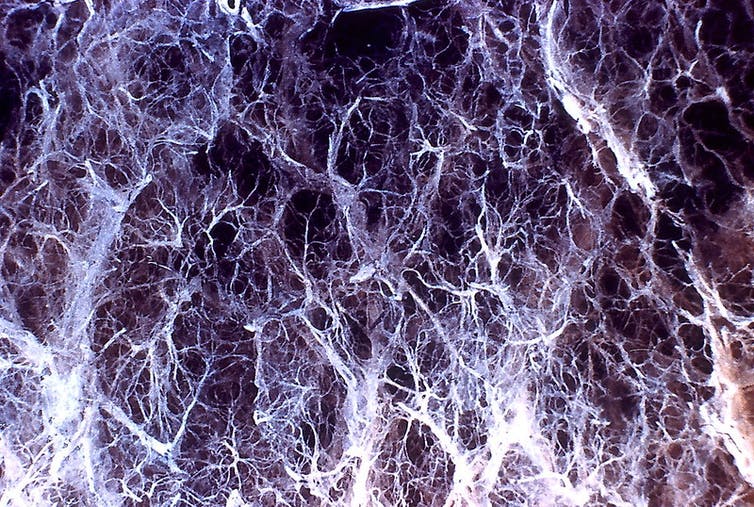
Lung damage from alpha-1 antitrypsin deficiency can lead to emphysema.
Atlas of Pulmonary Pathology/Flickr, CC BY-SA
But muscle cells aren’t very good at producing the AAT proteins the gene instructs them to make. Flotte and his team found that AAT levels one to five years after gene…