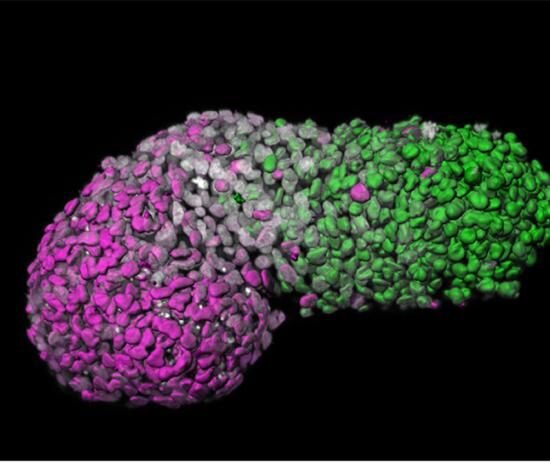
In a proof-of-concept study, scientists have demonstrated how 3D models made from embryonic stem cells, could be used as part of the testing process to assess if drugs and treatments are safe for developing embryos.
Before pharmaceuticals can be approved as safe for pregnant women, they must go through various stages of testing, including experiments to test whether drugs are safe during pregnancy in animal trials.
To complement these experiments, pharmaceutical companies are beginning to develop stem cell tests that might be able to predict outcomes earlier in the drug development pipeline. However, these stem cells are often arranged in single layers rather than the complex 3D arrangements which are seen in developing embryos.
Led by Crick group leader Naomi Moris during her time at the University of Cambridge, and in collaboration with researchers at Roche, scientists investigated the predictive potential of 3D models of embryonic stem cells called gastruloids. These models, when grown under precise conditions, organize themselves into similar arrangements as certain parts of developing embryos.
In their study, published in Reproductive Toxicology, the team tested the effects of seven pharmaceutical compounds, including ibuprofen, penicillin and thalidomide, on mouse and human gastruloids. They found that the gastruloids responded to the compounds in a similar way to known embryo reactions, providing evidence that they could eventually be adopted in pharmaceutical testing. Their use could help establish if compounds are likely to be safe or not, and could also improve the selection of which compounds to move forward into animal trials.
Naomi Moris, group leader of the Stem Cell & Human Development Laboratory at the Crick says, “Existing drug safety tests are robust and effective. But what gastruloids offer is a cell-based model which closely resembles the structure of embryos, that could help narrow down the number of compounds to move forward to animal tests.
“For drug developers, this could help better predict whether or not a potential new medicine might successfully make it through the lengthy trials process.”
The researchers will continue their work with gastruloids at the Crick, with more experiments to confirm these proof-of-concept results and to identify how best to scale up this method through assay development.
Scientists develop more accurate stem-cell model of early developing mouse embryo
More information:
Veronika Mantziou et al, In vitro teratogenicity testing using a 3D, embryo-like gastruloid system, Reproductive Toxicology (2021). DOI: 10.1016/j.reprotox.2021.08.003
Provided by
The Francis Crick Institute
Citation:
How embryo-like stem cell models could be used in drug safety tests (2021, August 25)
retrieved 25 August 2021
from https://phys.org/news/2021-08-embryo-like-stem-cell-drug-safety.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.