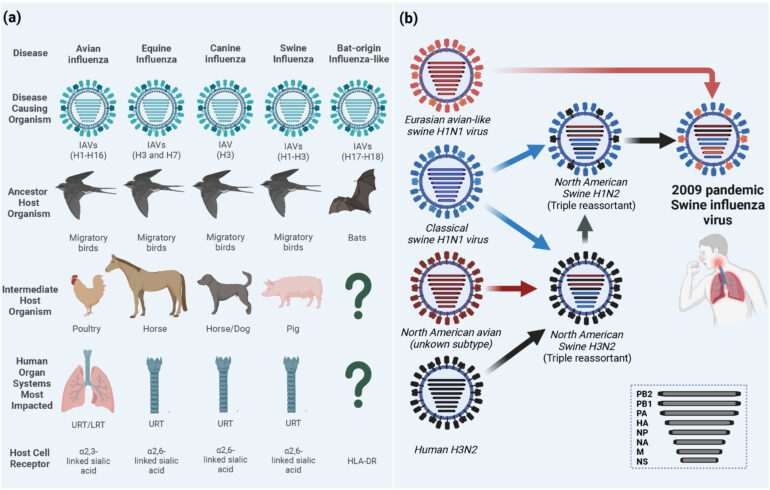
When the World Health Organization declared COVID-19 a pandemic on March 11, 2020, humans had been the only species with reported cases of the disease. While early genetic analyses pointed to horseshoe bats as the evolutionary hosts of SARS-CoV-2, the virus that causes COVID-19, no reports had yet surfaced indicating it could be transmitted from humans to other animal species.
Less than two weeks later, a report from Belgium marked the first infection in a domestic cat – presumably by its owner. Summer 2020 saw news of COVID-19 outbreaks and subsequent cullings in mink farms across Europe and fears of similar calls for culling in North America. Humans and other animals on and around mink farms tested positive, raising questions about the potential for a secondary wildlife reservoir of COVID-19. That is, the virus could infect and establish a transmission cycle in a different species than the one in which it originated.
Researchers have documented this phenomenon of human-to-animal transmission, colloquially referred to as spillback or reverse zoonotic transmission, in both domestic and wild animals. Wildlife may be infected either directly from humans or indirectly from domestic animals infected by humans. This stepping-stone effect provides new opportunities for pathogens to evolve and can radically change how they spread, as seen with influenza and tuberculosis.
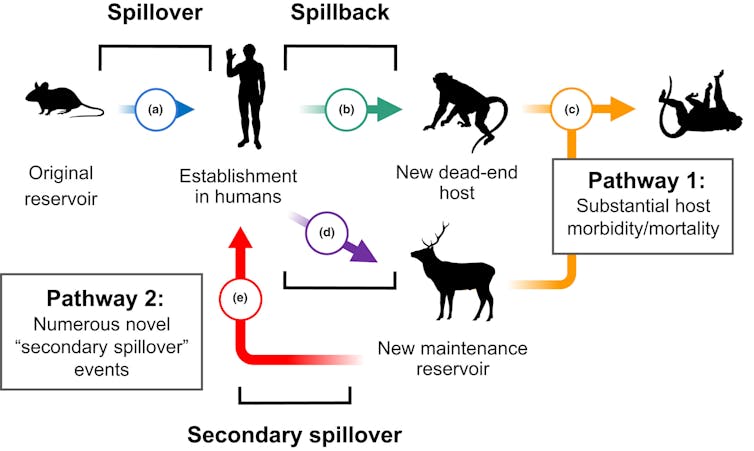
Pathogen transmission is bidirectional between animals and humans.
Fagre et al. 2022/Ecology Letters, CC BY-NC-ND
For example, spillback has been a long-standing threat to endangered great apes, even among populations with infrequent human contact. The chimpanzees of Gombe National Park, made famous by Jane Goodall’s work, have suffered outbreaks of measles and other respiratory diseases likely resulting from environmental persistence of pathogens spread by people living nearby or by ecotourists.
We are researchers who study the mechanisms driving cross-species disease transmission and how disease affects both wildlife conservation and people. Emerging outbreaks have underscored the importance of understanding how threats to wildlife health shape the emergence and spread of zoonotic pathogens. Our research suggests that looking at historical outbreaks can help predict and prevent the next pandemic.
Spillback has happened before
Our research group wanted to assess how often spillback had been reported in the years leading up to the COVID-19 pandemic. A retrospective analysis not only allows us to identify specific trends or barriers in reporting spillback events but also helps us understand where new emergent threats are most likely.
We examined historical spillback events involving different groups of pathogens across the animal kingdom, accounting for variations in geography, methods and sample sizes. We synthesized scientific reports of spillback across nearly a century prior to the COVID-19 pandemic – from the 1920s to…