Heart disease is the leading cause of death worldwide. The World Health Organization estimates that 17.9 million people lose their lives to it each year, accounting for 32% of global deaths.
Doris Taylor is a scientist working in regenerative medicine and tissue engineering. Her work has focused on creating personalized functioning human hearts in a lab that could rule out the need for donors. Taylor has dubbed these hearts “ghost hearts.”
In March, Taylor spoke at the 2023 Imagine Solutions Conference in Naples, Florida, about the ghost heart and her journey to creating it. Below are edited answers to questions from The Conversation. Taylor is also featured on Guy Kawasaki’s Remarkable People podcast.
What are the biggest challenges facing organ donations today?
Currently, patients in need of a heart transplant need to join a waitlist, and hearts become available when someone else has died. Because there are not enough hearts to go around, only the very sick are put on the waitlist. The U.S. transplants about 11 hearts a day, and on a given day there are more than 3,000 people waiting for a heart.
Even when organs are successfully transplanted, it isn’t a Hollywood fairy-tale ending. A person receiving an organ transplant essentially trades one disease for other medical complications and diseases. Toxic drugs necessary to prevent rejection can cause high blood pressure diabetes, cancer and kidney failure. These are serious medical issues that also affect people emotionally, financially and physically.
About 18% of people die in the first year after a transplant.
Doris Taylor speaks at the 2023 Imagine Solutions Conference.
What is the so-called “ghost heart”? How does it work?
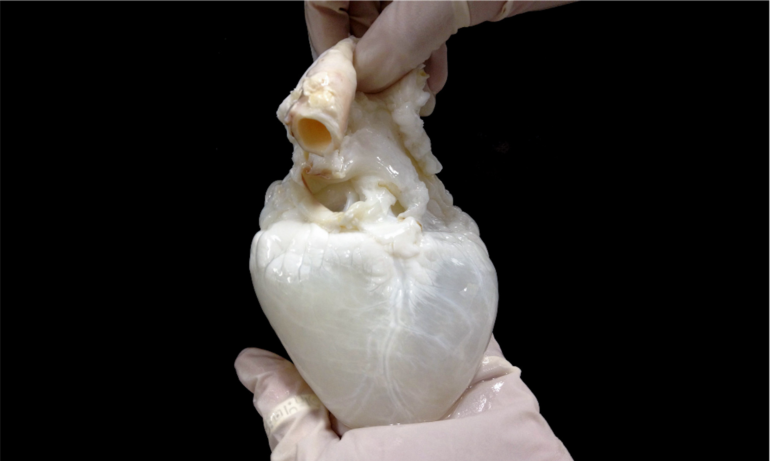
The ghost heart is a heart whose cells have been removed. All that remains is the heart framework, or scaffolding. It’s called a ghost heart because removing the cells causes the heart to turn from red to white. A human heart wouldn’t work as a scaffold because so few are available to work with.
So my team and I went with the next best thing: a pig heart. Pig hearts are similar to human hearts in terms of their size and structure. Both have four chambers – two atria and two ventricles – responsible for pumping blood. And structures from pig hearts such as valves have been used in humans safely.
To remove the cells, the pig heart is gently washed through its blood vessels with a mild detergent to remove the cells. This process is called perfusion decellurization. The cell-free heart can then be seeded with new cells – in this case, a patient’s cells – thus forming a personalized heart.
What role do stem cells play in creating a heart?
If you lined up the cells needed for an average-size 350-gram human heart, they would stretch for 41,000 miles. Stacked on top of one another, they would amount to 2 billion lines of cells, or enough to fill seven movie…