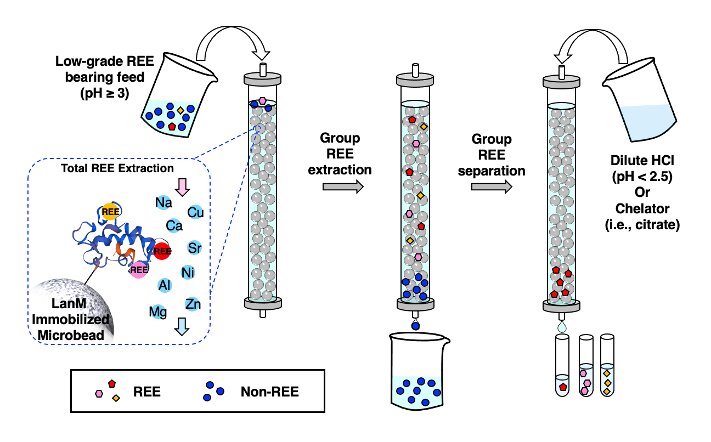
A new method improves the extraction and separation of rare earth elements—a group of 17 elements critical for technologies such as smart phones and electric car batteries—from unconventional sources. New research led by scientists at Penn State and the Lawrence Livermore National Laboratory (LLNL) demonstrates how a protein isolated from bacteria can provide a more environmentally friendly way to extract these metals and to separate them from other metals and from each other. The method could eventually be scaled up to help develop a domestic supply of rare earth metals from industrial waste and electronics due to be recycled.
“In order to meet the increasing demand for rare earth elements for use in emerging clean energy technologies, we need to address several challenges in the supply chain,” said Joseph Cotruvo Jr., assistant professor and Louis Martarano Career Development Professor of Chemistry at Penn State, a member of Penn State’s Center for Critical Minerals, and co-corresponding authors of the study. “This includes improving the efficiency and alleviating the environmental burden of the extraction and separation processes for these metals. In this study, we demonstrate a promising new method using a natural protein that could be scaled up to extract and separate rare earth elements from low-grade sources, including industrial wastes.”
Because the U.S. currently imports most of the rare earth elements it needs, a new focus has been placed on establishing a domestic supply from unconventional sources, including industrial waste from burning coal and mining other metals as well as electronic waste from cell phones and many other materials. These sources are vast but considered “low grade,” because the rare earths are mixed with many other metals and the amount of rare earths present is too low for traditional processes to work well. Furthermore, current methods for extraction and separation rely on harsh chemicals, are labor intensive, sometimes involve hundreds of steps, produce a high volume of waste, and are high cost.
The new method takes advantage of a bacterial protein called lanmodulin, previously discovered by the research team, that is almost a billion times better at binding to rare earth elements than to other metals. A paper describing the process appears online Oct. 8 in the journal ACS Central Science.
The protein is first immobilized onto tiny beads within a column—a vertical tube commonly used in industrial processes—to which the liquid source material is added. The protein then binds to the rare earth elements in the sample, which allows only the rare earths to be retained in the column and the remaining liquid drained off. Then, by changing the conditions, for example by changing the acidity or adding additional ingredients, the metals unbind from the protein and can be drained and collected. By carefully changing the conditions in sequence, individual rare earth elements could be separated.
“We first demonstrated that the method is exceptionally good at separating the rare earth elements from other metals, which is essential when dealing with low grade sources that are a hodgepodge of metals to start with,” said Cotruvo. “Even in a very complex solution where less than 0.1% of the metals are rare earths—an exceedingly low amount—we successfully extracted and then separated a grouping of the lighter rare earths from a grouping of the heavier rare earths in one step. This separation is an essential simplifying step because the rare earths have to be separated into individual elements to be incorporated into technologies.”
The research team separated yttrium (Y) from neodymium (Nd)—both abundant in primary rare earth deposits and coal byproducts—with greater than 99% purity. They also separated neodymium from dysprosium (Dy)—a crucial pairing that is common in electronic waste—with greater than 99.9% purity in just one or two cycles, depending on the initial metal composition.
“The high-purity of the recovered neodymium and dysprosium is comparable to other separation methods and was accomplished in as many or fewer steps without using harsh organic solvents,” said Ziye Dong, a postdoctoral researcher at LLNL and first author of the study. “Because the protein is able to be used for many cycles, it offers an attractive eco-friendly alternative to the methods currently used.”
The researchers do not think their method will necessarily supplant the current liquid-liquid extraction process that is commonly used for high-volume production of lighter rare earth elements from high-grade sources. Instead, it will allow for efficient use of low-grade sources and especially for extraction and separation of the rarer and generally far more valuable heavy rare earths.
“Other recent methods are capable of extracting rare earth elements from low-grade sources, but they typically stop at a ‘total’ product that has all the rare earths lumped together, which has relatively little value and then needs to be funneled into more conventional schemes for further purification of individual rare earth elements,” said Dan Park, staff scientist at LLNL and co-corresponding author of the study. “The value is really in the production of individual rare earths and especially the heavier elements.”
“Our process is particularly convenient because these high-value metals can be purified off the column first,” added Cotruvo.
The researchers plan to optimize the method so fewer cycles are required to obtain the highest-purity products and so it can be scaled up for industrial use.
“If we can engineer derivatives of the lanmodulin protein with greater selectivity for specific elements, we could recover and separate all 17 rare earth elements in a relatively small number of steps, even from the most complex mixtures, and without any organic solvents or toxic chemicals, which would be a very big deal,” said Cotruvo. “Our work shows that this goal should be achievable.”
New sensor detects valuable rare earth element terbium from non-traditional sources
More information:
Ziye Dong et al, Bridging Hydrometallurgy and Biochemistry: A Protein-based Process for Recovery and Separation of Rare Earth Elements, ACS Central Science (2021). DOI: 10.1021/acscentsci.1c00724
Provided by
Pennsylvania State University
Citation:
New, environmentally friendly method to extract and separate rare earth elements (2021, October 8)
retrieved 10 October 2021
from https://phys.org/news/2021-10-environmentally-friendly-method-rare-earth.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.