A large international study in hospitalized patients with severe COVID-19 has shown that while a higher dose of steroids did not significantly reduce mortality, there was a trend towards benefit without increased side effects.
Published today in the Journal of the American Medical Association, the study compared the standard 6mg dose of the steroid dexamethasone with 12mg in patients requiring a higher degree of support to maintain their oxygen levels. It is the first COVID-19 trial to report on long term mortality and one of only a few that were ‘blinded,” or designed in a way so that doctors didn’t know which patient received which dose.
Study co-author Professor Bala Venkatesh from The George Institute for Global Health said that although previous research suggested higher doses of dexamethasone may benefit patients with more severe COVID-19, there had been concerns about potential adverse reactions.
“Our study found no differences in the adverse event rates between the two doses. While there had been reports of infections with so called ‘black fungus’ in patients with COVID-19 with a weakened immune system, our study found no increase in infection rates with the higher doses of steroids,” he said.
Patients with critical COVID-19 typically have severe lung inflammation and very low oxygen levels, which often leads to the requirement for increasing oxygen support, mechanical ventilation, support to maintain blood pressure and kidney dialysis. Dexamethasone is the mainstay of treatment for severe COVID-19. While a 6mg daily dose is recommended for up to ten days, there have been indications that a higher dose may benefit those with more severe disease. A potential side-effect of steroid use is suppression of the body’s immune system, diminishing the ability to fight other types of infections. There have been some reports of steroid-treated COVID-19 patients developing serious fungal infections, known as mucomycosis or “black fungus.”
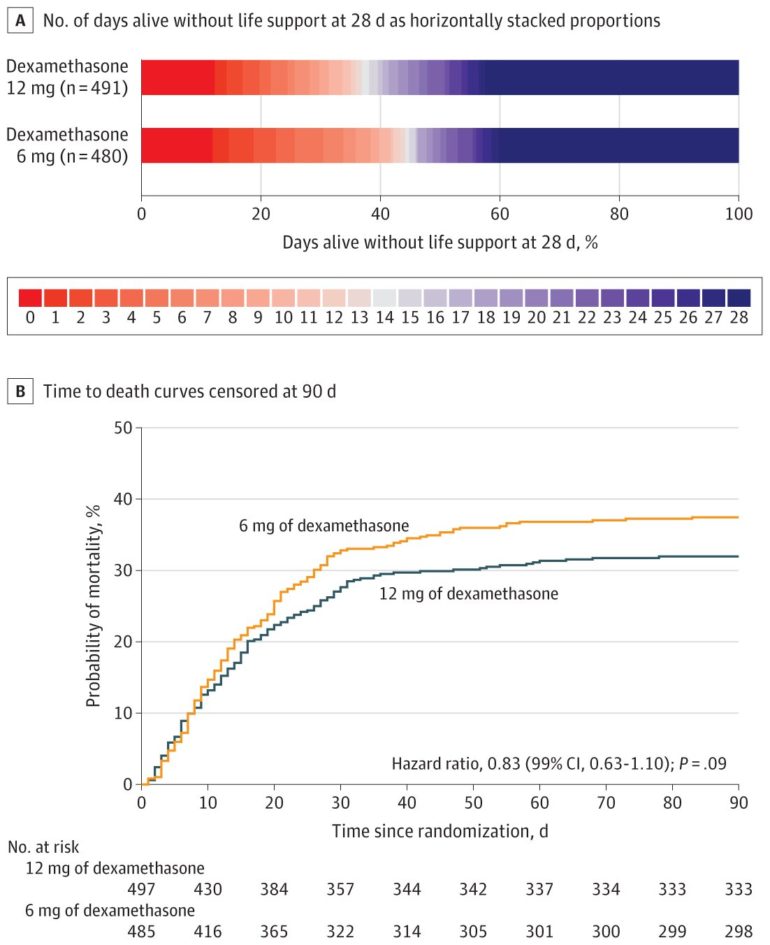
In this study, researchers recruited 1000 adult patients with confirmed SARS-CoV-2 infection across 31 sites in 26 hospitals in Denmark, India, Sweden, and Switzerland between August 2020 and May 2021. The patients were randomly assigned into two groups, one receiving intravenous dexamethasone 12 mg (n=503) and the other dexamethasone 6 mg daily (n=497) for up to ten days. The proportion of patients alive and not requiring life support after 28 days were 42.6 percent in the 12mg group and 40.2 percent in the 6mg group. The death rates were 27.1 percent and 32.3 percent in patients assigned to 12mg and 6mg group, respectively.
The George Institute India’s Professor Vivek Jha, who along with Prof Venkatesh set up and conducted the trial in the 12 participating hospitals in India, said that the study provided useful guidance to clinicians treating COVID-19 patients around the world.
“Whilst the data do not provide unequivocal evidence that dexamethasone 12mg is better than 6 mg, we saw a trend towards reduced requirement for life support and mortality at the higher dose without any increase in risk of serious infections,” he said. “As dexamethasone is cheap, easily available and indicated for the treatment of COVID-19 patients with critically low oxygen levels, even a small difference in death rates or health outcomes could lead to important clinical and health economic benefits at the population level.”
European drugs agency endorses steroid for COVID treatment
More information:
Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia, JAMA (2021). DOI: 10.1001/jama.2021.18295
Provided by
George Institute for Global Health
Citation:
Patients with severe COVID-19 could benefit from higher doses of corticosteroids (2021, October 22)
retrieved 24 October 2021
from https://medicalxpress.com/news/2021-10-patients-severe-covid-benefit-higher.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.