Quantum electrodynamics is the best-tested theory in physics. It describes all electrical and magnetic interactions of light and matter. Scientists at the Max-Planck-Institut für Kernphysik in Heidelberg (MPIK) have now used precision measurements on their Alphatrap experiment to investigate the magnetic properties of electrons bound to highly ionized tin atoms. Such tests provide insights into the behavior of particles under extreme field strengths. They also serve as a starting point for the search for new physics.
Like other charged elementary particles, the electron has a magnetic moment. Its exact value is one of the most precisely known measurements in physics and is in excellent agreement with the theoretical predictions of quantum electrodynamics. Theory and experiment are in agreement to more than ten decimal places. However, these values apply only to the “free” electron, which is not subject to external fields.
But especially in the case of very strong fields, such as those present in the immediate vicinity of atomic nuclei, additional factors could come into play—such as previously unknown elementary particles or deviations from the known laws of nature.
A research team combining scientists from several divisions at MPIK has now achieved an important milestone here. “We have succeeded in producing hydrogen-like tin ions and storing them for months in the Alphatrap ion trap,” says Jonathan Morgner, first author of the new study and Ph.D. student in Professor Klaus Blaum’s division. The experiment is led by Sven Sturm, and the theory group led by Zoltán Harman from Christof Keitel’s division performed the calculations.
Morgner continues, “Thanks to the long storage time, we were able to measure the magnetic moment with unprecedented precision.” The study appeared in Nature.
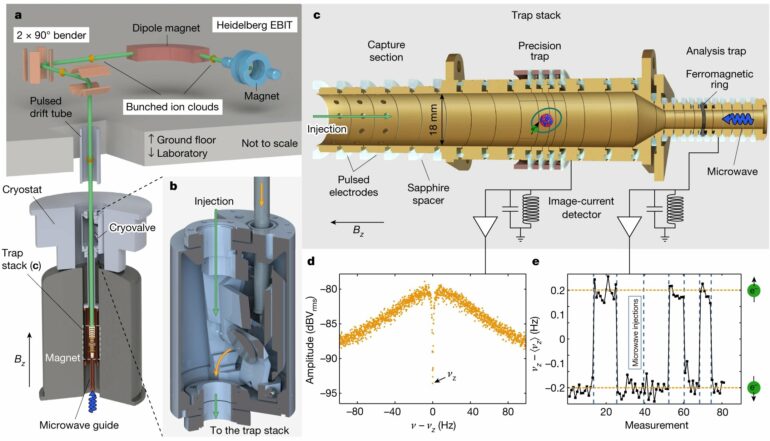
Hydrogen-like tin has only one electron in its shell—just like conventional hydrogen. However, the atomic nucleus of tin has 50 protons, and the neutral element consist of 50 electrons in its shell. “So we first had to remove 49 electrons,” Morgner says. “To achieve this, we used an electron beam ion trap, the Heidelberg-EBIT, which is a device for generating highly charged ions, developed by José Crespo López-Urrutia from the division of Thomas Pfeifer.”
In this experimental setup, a cloud of about 100,000 tin ions is bombarded with high-energy electrons. In the process, the ions successively lose their bound electrons. Afterwards the ions that have only one electron left in their shells are filtered and fed into the particle trap of the Alphatrap experiment. There, the magnetic properties of the electrons are measured.
Alphatrap is a high-precision experiment whose heart is a Penning trap, in which charged particles are held in place with electromagnetic fields. It also has a cryogenic vacuum system that uses low temperatures to create an extremely good vacuum. This is necessary as the highly charged tin ions would immediately acquire electrons from any surrounding atoms. This would make longer-term measurements impossible. In addition, the preparation of hydrogen-like tin is very time-consuming.
The researchers were then able to measure the so-called g factor of the electron on the captured tin ions using irradiated microwaves. At the matching frequency, the electrons in the applied magnetic field in the trap make so-called spin flips—i.e., a reorientation of their “magnetic needle.” This effect allows highly accurate measurements of the g factor, also called “gyromagnetic factor.” This is a measure of how strong the magnetic field of the electron is.
The dimensionless value of the g factor is about 2. The exact value can be predicted by quantum electrodynamics, and depends on the environment of the electron. And this is where the highly charged tin ions come into play. Since they have only one electron, they can be described in theory similarly to a hydrogen atom.
This simplifies the calculations enormously. However, due to the high charge of the tin nucleus, extremely high electric fields of around 1015 volts per centimeter are present on the position of the electron around the atomic nucleus. These field strengths are orders of magnitude stronger than what can be realized today even with the most powerful laser systems in any experiments. Thus, such atomic nuclei are ideally suited to test the predictions of quantum electrodynamics especially in the high-field regime, i.e., under extreme conditions.
The complicated calculations of the theoretical value of the g factor at strong fields provide somewhat less precise predictions than for the free electron, due to the additional interaction with the nucleus. According to theory, the g factor should be gtheo = 1.910 561 821 (299). The experimental value measured in the Alphatrap apparatus has much higher accuracy and is gexp = 1.910 562 058 962 (73)stat (42)sys (910)ext.
“The two values are in very good agreement, so this is an excellent confirmation of quantum electrodynamics,” Morgner said. “This shows that our previous understanding of physics works even at such extreme fields.” Until now, comparable measurements of the g factor of electrons had only been performed for much lighter elements such as silicon.
Thanks to the successful measurements, the next step would be to experiment with even heavier ions. Hydrogen-like lead or uranium have a much higher electric field than tin. Further upgrades are currently being implemented to enable further and even stronger fundamental tests of quantum electrodynamics in the future.
More information:
Jonathan Morgner, Stringent test of QED with hydrogen-like tin, Nature (2023). DOI: 10.1038/s41586-023-06453-2. www.nature.com/articles/s41586-023-06453-2
Provided by
Max Planck Society
Citation:
A precise test of quantum electrodynamics: Measuring the g factor of electrons in hydrogen-like tin (2023, October 4)