Preservation of the high-pressure states of materials at ambient conditions is a long-sought-after goal for fundamental research and practical applications.
A team of scientists led by Drs. Zhidan (Denise) Zeng, Qiaoshi Zeng, and Ho-Kwang Mao from the Center for High Pressure Science and Technology Advanced Research (HPSTAR) and Prof. Wendy Mao from Stanford University report an innovative breakthrough where they were able to maintain the extraordinary properties of high-pressure materials in free-standing, nanostructured diamond capsules without the support of traditional bulky pressure vessels. Their work was recently published in Nature.
Modern technology is built upon access to materials with suitable physical and chemical properties that can be used to perform specific functions in various devices. Technological advances, therefore, are often dictated by the development of superior materials with desirable properties. High pressure can drastically alter or tune properties of all materials, thus providing a fertile ground for discovering novel materials with extremely favorable properties.
The caveat is, however, that the favorable properties often only exist under pressure when the sample remains in the bulky high-pressure vessel, limiting scientific investigation and potential applications. For the past century, scientists have tried to overcome this difficulty. They succeeded only in “quenchable” phases, where novel materials synthesized at high pressure retain their favorable properties after releasing pressure. A well-known example is the high-pressure conversion of ordinary carbon into diamond which is able to keep its brilliance and other exceptional properties after retrieving at ordinary pressures.
Unfortunately, such successful examples of quenchable phases are extremely rare, largely rendering high-pressure materials studies of only academic interest with little practical value in the ambient environment.
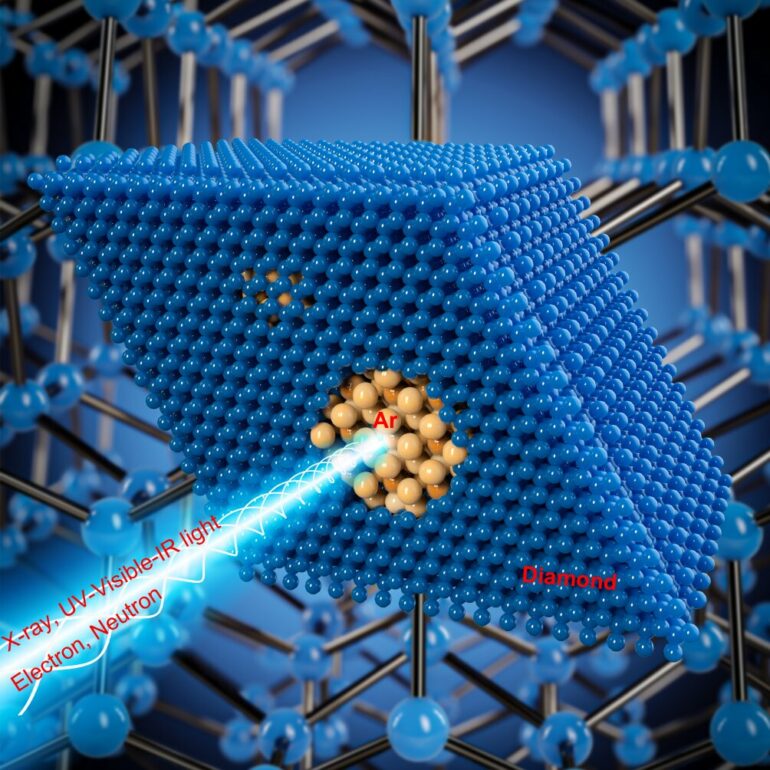
The HPSTAR and Stanford research group developed a novel approach that has demonstrated the ability to quench even tenuous gases and preserve their high-pressure properties. They compressed glassy carbon, an amorphous form of porous carbon, together with argon gas to 50 gigapascals—about 500,000 times atmospheric pressure, and heated the sample to 3,320 degrees Fahrenheit.
The glassy carbon that is initially impermeable to gases at ordinary conditions absorbs argon like a sponge at high pressures. The application of high pressure and temperature conditions converts the carbon into diamond and traps the now solid, high-pressure argon in its pores. The resulting sample that is