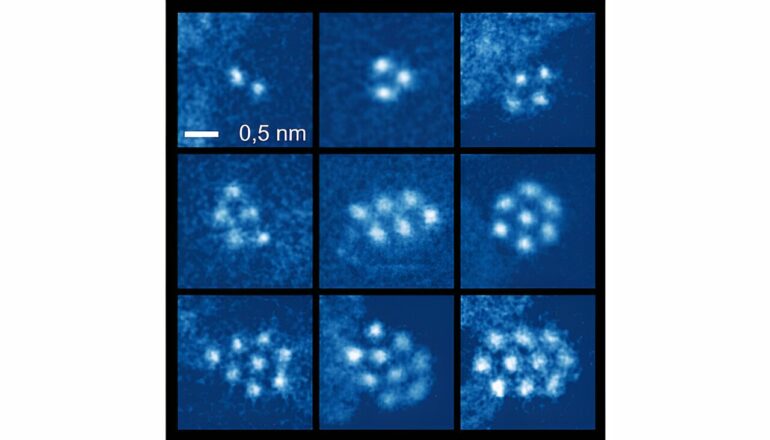
For the first time, a research team has succeeded in stabilizing and directly imaging small clusters of noble gas atoms at room temperature. This achievement opens exciting possibilities for condensed matter physics and applications in quantum information technology.
The key to this breakthrough, achieved by researchers at the University of Vienna in collaboration with colleagues at the University of Helsinki, was the inclusion of noble gas atoms between two graphene layers. This overcomes the difficulty that noble gases do not form stable structures under experimental conditions at room temperature.
Details of the method and the first electron microscopic images of noble gas structures (krypton and xenon) have now been published in Nature Materials .
Jani Kotakoski’s group at the University of Vienna was studying the use of ion irradiation to change the properties of graphene and other two-dimensional materials when they noticed something unusual: When noble gases are used for irradiation, they can become trapped between two graphene layers. This happens when the noble gas ions are fast enough to penetrate the first but not the second graphene layer.
Once trapped between the layers, the noble gases can move freely. This is because they do not form chemical bonds. However, to accommodate the noble gas atoms, the graphene bends and forms tiny bubbles. Here, two or more noble gas atoms can meet and form regular, tightly packed, two-dimensional noble gas nanoclusters.
“We observed these clusters using scanning transmission electron microscopy, and it is really fascinating and a lot of fun to observe them. They rotate, jump, grow and shrink as we image them,” says Manuel Längle, lead author of the study. “Getting the atoms between the layers was the hardest part of the work. Now that we’ve done that, we have a simple system for studying fundamental processes related to the growth and behavior of materials,” he adds.
Regarding the group’s future work, Kotakoski says, “The next steps are to study the properties of clusters with different noble gases and how they behave at low and high temperatures. Due to the use of noble gases in light sources and lasers These new structures will enable future applications, for example in quantum information technology.”
More information:
Manuel Längle et al, Two-dimensional few-atom noble gas clusters in a graphene sandwich, Nature Materials (2024). DOI: 10.1038/s41563-023-01780-1
Provided by
University of Vienna
Citation:
First direct imaging of tiny noble gas clusters at room temperature (2024, January 11)