With their unique appearance, texture, and mouthfeel, fondants have intrigued bakers and physicists for years. They present an appetizing enigma in the world of confectionery, an intriguing combination of sugar, water, and heat that, when manipulated correctly, yields a delectably creamy product.
Researchers from the Max Planck Institute for Polymer Research and Technische Universität Berlin studied the kinetic and thermodynamic processes of sugar crystallization in the making of fondant.
They combined a controlled kneading machine with light microscopy to precisely observe the process of fondant creation and link it to theoretical physics models. This allowed the team to track how different preparation methods influence the final structure and texture of fondant. The article, “Crystallization in highly supersaturated, agitated sucrose solutions,” was published in Physics of Fluids.
Such work is poised to provide new ways to better predict and control fondant texture, mouthfeel, and flow process.
“Our works shows the interplay between crystallization speeds and process parameters. Thus, we can predict structure and function,” said author Hannah Hartge. “So far, food systems like fondants were rarely explored in scientific research, especially physics.”
Historically, it has been difficult to reproducibly research thicker sugar solutions because they crystalize under cooling and stirring. To address this challenge, the group required a method with more control than a standard kitchen.
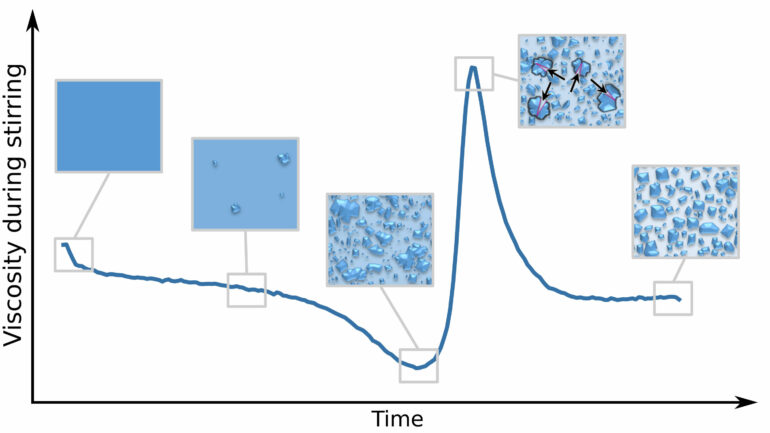
To study fondant creation, the researchers used a laboratory kneader, which allowed them to observe how fondants are produced and how they crystallize under high-force stirring.
As the kneading progressed and more sugar was pulled out of solution into crystals, the torque of the kneader dropped, which indicates a smoother texture. With further kneading, however, the large sugar crystals began to break up, leading to a peak in torque and resulting in a thicker solution more characteristic of a fondant.
“It was surprising to see the sugar crystals in the solution grow first during the early stages of stirring, and then the biggest get smaller again due to the stirring,” said author Thomas Vilgis. “Finally, their sizes adjust themselves, which leads to this fine creamy texture of fondants, provided the concentration, temperatures, and stirring speed are chosen correctly.”
This peak in thickness was highly dependent on the temperature and stirring speed, mimicking a well-established model—called classical nucleation theory—for how dissolved particles glom onto each other.
In future work, Hartge and Vilgis hope to explore the behavior of fondants created with sugar alternatives, such as erythritol and isomalt, paving the way for healthier, low-calorie fondant options. They aspire to inspire more studies on food systems from a fundamental physics perspective.
More information:
Crystallization in highly supersaturated, agitated sucrose solutions, Physics of Fluids (2023). DOI: 10.1063/5.0150227
Provided by
American Institute of Physics
Citation:
Fondant: Where baking and thermodynamics mix (2023, June 27)