Researchers at Duke University have uncovered the molecular inner workings of a material that could underpin next-generation rechargeable batteries.
Unlike today’s popular lithium-ion batteries that feature a liquid interior, the lithium-based compound is a solid at operational temperatures. But despite its rigid interior structure, charged ions are still able to quickly travel through, making it a “super ionic” material. While researchers have been interested in this compound for some time, they have not known how lithium ions are able to pass through its solid crystalline structure so easily.
The new results answer many standing questions, showing surprising liquid-like behavior at the atomic level. With these insights in hand, as well as the machine learning models used to obtain them, researchers are set to explore similar recipes to solve many of the field’s long-standing challenges.
The results were published online January 6 in the journal Nature Physics.
In general, rechargeable batteries work by moving atoms with an electric charge called ions through a chemical substance called an electrolyte. How quickly and easily these ions can make their journey plays a key role in how fast a battery can charge and dispense energy.
Lithium-ion batteries have long been the dominant technology used for most commercial applications, from tiny smart watches to gigantic grid energy storage facilities. While they have been extremely successful, lithium-ion batteries have several drawbacks that make new technologies more attractive for certain applications.
For example, lithium-ion batteries normally have a liquid electrolyte that, while extremely efficient at allowing lithium ions to travel quickly, is also dangerously flammable. This liquid electrolyte is also corrosive, limiting the choices engineers can make in designing other critical components of the battery.
“There is a huge effort going on to design better batteries so that we can have electric cars that drive farther, charge faster and are safer from unexpected fires,” said Olivier Delaire, associate professor of mechanical engineering and materials science at Duke. “Solid-state batteries are extremely attractive because of their stability, but figuring out how to design them so ions can flow through them quickly is a fundamental question in the field.”
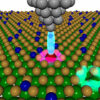
One potential solid-state electrolyte researchers are focusing on is a form of argyrodite based on lithium. Already identified as a superionic material, it walks the line between a crystalline and liquid state of matter. While it is a solid at room temperatures, it still allows lithium ions to travel as quickly through its molecular structure as its liquid cousins. How and why these ions are able to do this, however, has long remained a mystery.
To solve this lingering question, Delaire and his colleagues investigated samples with the material at the Spallation Neutron Source at Oak Ridge National Laboratory. By bouncing neutrons off the atoms at extremely fast rates, researchers captured a series of snapshots of the atoms’ precise motions inside the atomic scaffold of the argyrodite compound.
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights.
Sign up for our free newsletter and get updates on breakthroughs,
innovations, and research that matter—daily or weekly.
“Working with this material was a little bit trickier than usual because lithium tends to absorb a lot of neutrons, making this sort of investigation difficult,” Delaire said. “We had to lean on our collaborators from the University of Münster to obtain a rare variety with an extra neutron called Lithium 7 to get the data.”
Once the atomic dynamics were measured, Delaire’s team was granted time at the National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory to make sense of it all. After running simulations and models that take weeks even on these incredibly powerful computers, the team was able to compare their computational results with the measurements they observed.
The researchers discovered that the ions’ ability to traverse this material quickly stems from its molecular vibrations. At lower temperatures, the molecular structures within solids tend to vibrate with a smaller number of intrinsic frequencies, making it difficult for ions to move through them. But this form of lithium argyrodite instead has a larger number of vibration modes like a liquid.
One can think of this like a basketball team trying to move the ball around through an offensive play. If players are generally just standing around and not very active, it’s harder for them to pass the ball through the defense. But if all the players are active and moving around, it’s easier for the ball to find paths between them.
“Gaining an understanding of how all of this works will help us design materials based on these principles,” Delaire said. “For example, with these ideas in mind, the materials don’t necessarily need to have lithium in them. Lithium is expensive and there aren’t that many places that produce it, so if we could design similar materials based on sodium, for example, these batteries could become much cheaper and easier to produce.”
More information:
Jingxuan Ding et al, Liquid-like dynamics in a solid-state lithium electrolyte, Nature Physics (2025). DOI: 10.1038/s41567-024-02707-6
Citation:
Liquid-like molecular dynamics explain solid-state battery material’s superionic transport abilities (2025, January 7)