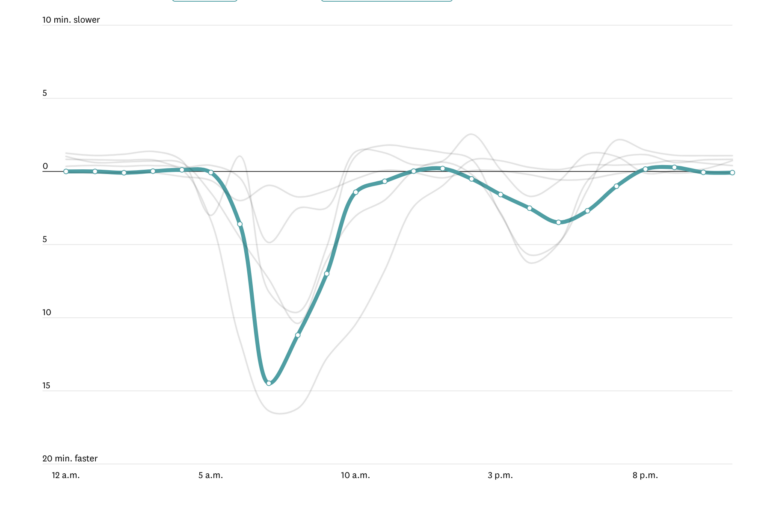
The word magic is not often used in the context of science. But in the early 1930s, scientists discovered that some atomic nuclei – the center part of atoms, which make up all matter – were more stable than others. These nuclei had specific numbers of protons or neutrons, or magic numbers, as physicist Eugene Wigner called them.

Maria Goeppert Mayer won the 1963 Nobel Prize in physics.
Argonne National Laboratory, CC BY-NC-SA
The race to figure out what made these nuclei so stable began. Understanding these magic numbers would allow scientists to predict the properties of other nuclei, such as their mass or how long they are expected to live. With that, scientists could also predict which combinations of protons and neutrons can result in a nucleus.
The solution to the puzzle came in 1949 from two directions simultaneously. In the U.S., physicist Maria Goeppert Mayer published an explanation, at the same time as a group of scientists led by J. Hans D. Jensen in Germany found the same solution.

Hans Daniel Jensen won the 1963 Nobel Prize in physics.
The Nobel Foundation
For their discovery, the two physicists each got a quarter of the 1963 Nobel Prize in physics. We’re two nuclear scientists whose work is built on Goeppert Mayer’s and Jensen’s discoveries 75 years ago. These magic numbers continue to play an important role in our research, only now we can study them in nuclei that live for just a fraction of a second.
Stability in the atom
The atom is a complex system of particles. It’s made up of a central nucleus consisting of protons and neutrons, called nucleons, with electrons orbiting around the nucleus.
Nobel prize-winning physicist Niels Bohr described these electrons in the atom as existing in a shell structure. The electrons circulate around the nucleus in particular energy levels, or orbits. These orbits have specific energies, and each orbit can hold only so many electrons.
Chemical reactions result from interactions between the electrons in two atoms. In Bohr’s model, if an electron orbit is not already filled, then it’s easier for the atoms to exchange or share those electrons and induce chemical reactions.
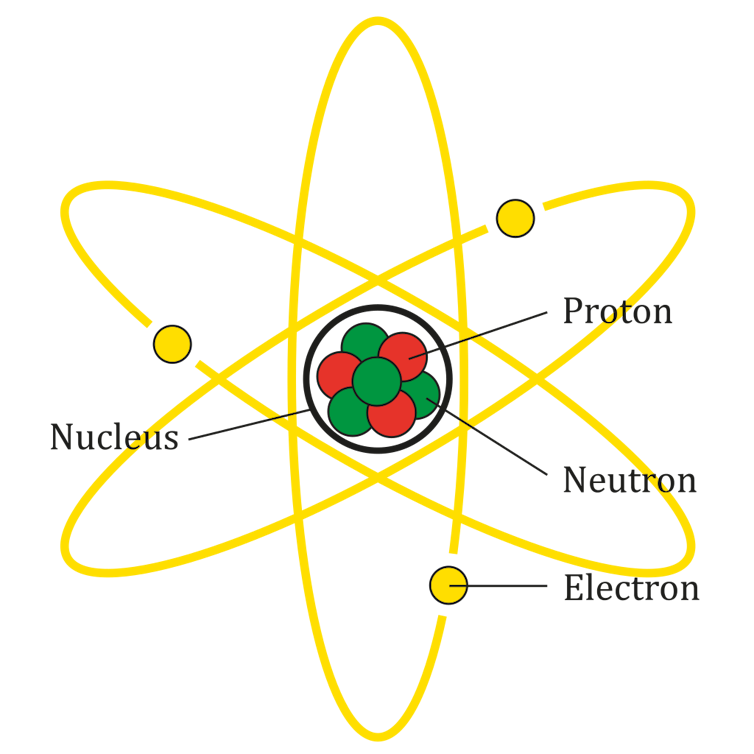
The Bohr model of the atom.
AG Caesar/Wikimedia Commons, CC BY-SA
One class of elements, the noble gases, hardly ever react with other elements. In noble gases, the electrons occupy completely filled orbits, and as a result the atoms greedily hold onto their electrons instead of sharing and undergoing a chemical reaction.
In the 1930s, scientists wondered whether protons and neutrons might also occupy orbits, like electrons. But nobody could show this conclusively. For more than a decade, the scientific community was unable to describe the nucleus in terms of individual protons and neutrons. Scientists used a more simplified picture, one that treated protons and neutrons as one…