If you imagine yourself peering through a microscope, you probably picture looking at a glass slide with an amoeba, or maybe a human cell, or perhaps even a small insect of some kind.
But microscopes can see much more than these small living things, and a new type of microscopy developed at Caltech is making it easier to see the very molecules that make up living things.
In a paper appearing in the journal Nature Photonics, researchers from the lab of Lu Wei, assistant professor of chemistry and investigator with the Heritage Medical Research Institute, demonstrate what they are calling bond-selective fluorescence-detected infrared-excited spectro-microscopy, or BonFIRE.
BonFIRE combines two microscopy techniques into one process with greater selectivity and sensitivity, enabling researchers to visualize biological processes at the unprecedented single-molecule level and understand biological mechanisms from a molecular point of view.
“With our new microscope, we can now visualize single molecules with vibrational contrast, which is challenging to do with existing technologies.” says Dongkwan Lee, study co-author and chemical engineering graduate student.
One technique involved in BonFIRE is fluorescence microscopy, which images molecules and other microscopic structures by tagging them with fluorescent chemical markers, causing them to glow when imaged.
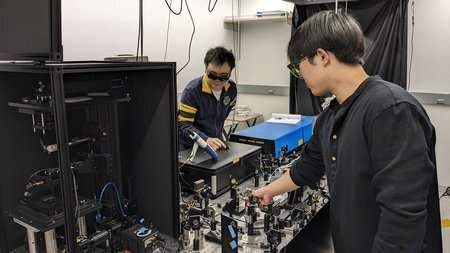
Postodoctoral scholar Haomin Wang (left) and graduate student Dongkwan Lee (right) demonstrate operation of the BonFIRE microscopy apparatus. © Caltech
The other technique is vibrational microscopy, which makes use of natural vibrations in the bonds that hold together the atoms of a molecule. A sample to be imaged is bombarded with light, in this case infrared light. That bombardment causes the bonds of the material’s molecules to vibrate in such a way that their type can be identified. Vibrations of a triple bond will “sound” different than the vibrations of a single bond, and the vibrations of a carbon atom bonded to another carbon atom will sound different than the vibrations of a carbon atom bonded to a nitrogen atom, for example. It’s not unlike how a trained guitarist would be able to tell which string on a guitar was plucked and what material it is made from just by listening to the tone it makes.
Wei says that fluorescence microscopy allows researchers to observe single molecules, but it does not provide rich chemical information. On the other hand, vibrational microscopy provides that rich chemical information but only works when the molecule being imaged is present in large amounts.
BonFIRE gets around these limitations by coupling vibrations to fluorescence, effectively combining the strengths of the two techniques. The process works like this: The sample is first stained with a fluorescent dye that bonds to the molecules intended to be imaged. The sample is then bombarded by a pulse of infrared light, whose frequency is tuned to excite a specific bond found in that dye. Once the bond is excited by just a single photon of that light, a second higher-energy pulse of light shines on it and excites it to fluoresce with a glow that can be detected by the microscope. In this way, the microscope can image entire cells or single molecules.
“We are fascinated by this spectroscopy process and are excited to turn it into a novel tool for modern bioimaging,” says Haomin Wang, study co-author and postdoctoral scholar research associate in chemistry. “Over the past three years, we have been on an adventure to build our custom BonFIRE microscope and gain deeper understanding on this spectroscopic process, which further helped us to optimize each component in our setup to reach the performance we have now.”
In their paper, the researchers also demonstrate the ability to tag biomolecules with “colors,” allowing them to be differentiated from each other. This is done by using several isotopes of the atoms that make up the dye molecule. (Isotopes are forms of an element with different atomic weights because their nuclei have greater or fewer neutrons). The frequency at which their bonds vibrate changes with the increased or decreased mass of the atoms.
“Unlike conventional fluorescence microscopy, which can only distinguish a handful of colors at a time, BonFIRE uses infrared light to excite different chemical bonds and produces a rainbow of vibrational colors,” Wei says. “You can label and image many different targets from the same sample at a time and reveal the molecular diversity of life in stunning detail. We hope to be able to demonstrate the imaging capability with tens of colors in live cells in the near future.”
Additional co-authors are chemistry graduate students Yulu Cao, Xiaotian Bi, Jiajun Du, and Kun Miao.
More information:
Haomin Wang et al, Bond-selective fluorescence imaging with single-molecule sensitivity, Nature Photonics (2023). DOI: 10.1038/s41566-023-01243-8
Provided by
California Institute of Technology
Citation:
Microscopy techniques combine to create more powerful imaging device (2023, June 29)