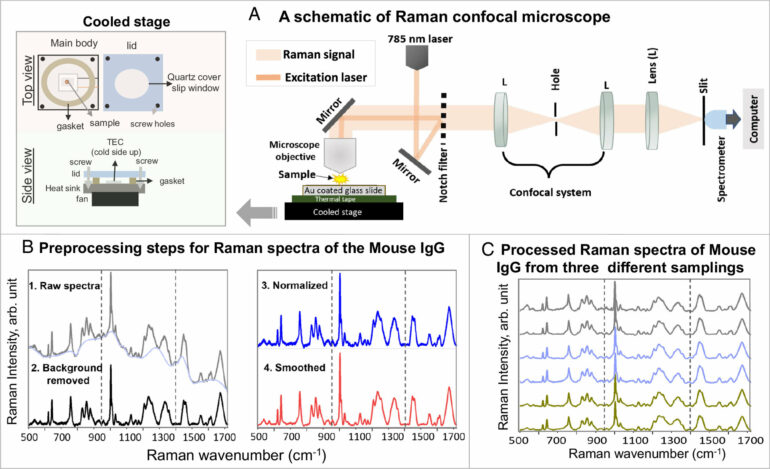
Raman spectroscopy—a chemical analysis method that shines monochromatic light onto a sample and records the scattered light that emerges—has caused frustration among biomedical researchers for more than half a century. Due to the heat generated by the light, live proteins are nearly destroyed during the optical measurements, leading to diminishing and non-reproducible results. As of recently, however, those frustrations may now be a thing of the past.
A group of researchers with the Institute for Quantum Sciences and Engineering at Texas A&M University and the Texas A&M Engineering Experiment Station (TEES) have developed a new technique that allows low-concentration and low-dose screenings of protein-to-ligand interactions in physiologically relevant conditions.
Titled thermostable-Raman-interaction-profiling (TRIP), this new approach is a paradigm-shifting answer to a long-standing problem that provides label-free, highly reproducible Raman spectroscopy measurements. The researchers published their findings in the Proceedings of the National Academy of Sciences.
“Protein is a very fragile biological molecule and needs specific care,” said lead author and postdoctoral research assistant Dr. Narangerel Altangerel. “When I cool down the surface or substrate, I can make the proteins happy. I can poke them with the laser, and they can now output the information I need.”
While the proteins studied are on a molecular level, the implications of these findings could be huge. Like a lock and key, a protein-ligand interaction is the first step in processes like signal transduction, immune responses and gene regulation. Due to TRIP’s ability to detect protein-ligand interactions in real time, the timeline for drug and vaccine testing may be shortened. Another application could be clinical, turning lengthy tests to detect a virus into same-day turnaround with accurate results.
“The whole idea from the spectroscopy statute is that it requires minimum to none for sample preparation, so this can be moved into the clinic right away,” said co-author and University Professor in the Department of Biomedical Engineering Dr. Vladislav Yakovlev. “Clinicians and patients don’t have to wait for days and weeks of analysis. You can get all these answers almost right away.”
An additional benefit of the TRIP technique is that the sample size required to run the test is much smaller and requires a lower protein concentration, meaning a more cost-effective process for testing.
“I was buying 100 microliters of a sample for $3,500, then I have to share this sample with multiple people and end up with only a 20 to 30 microliter sample,” Altangerel said. “This forced me to use a smaller sample making Raman hard to do, as low-concentration samples make it a weak process. That made me challenge myself to try different things.”
Despite the breakthrough, the team is looking for other aspects in which the TRIP method could be useful.
“In a follow-up article, we are trying to identify the chemical composition of those proteins just using this technique so we can apply this to similar ideas related to DNA analysis and other biological molecules,” Yakovlev said. “Something that normally requires sequencing but utilizes TRIP, so you don’t need any sample preparation.”
“For a long time. people thought this is impossible to do,” Dr. Yakovlev said. “But Dr. Altangerel demonstrated that nothing, indeed, is impossible if you do things right.”
More information:
Narangerel Altangerel et al, Label-free drug interaction screening via Raman microscopy, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2218826120
Provided by
Texas A&M University College of Engineering
Citation:
Novel Raman technique breaks through 50 years of frustration (2023, July 26)