In the search for more efficient and sustainable energy generation methods, a class of materials called metal halide perovskites have shown great promise. In the few years since their discovery, novel solar cells based on these materials have already achieved efficiencies comparable to commercial silicon solar cells.
Yet, perovskite solar cells offer significant advantages over silicon: Their manufacturing and energy costs are lower, as they can be produced using cost-effective coating processes. Moreover, their flexibility and lightweight nature allows for application on a wide range of surfaces, from portable electronics to innovative building facades.
But how does a solar cell actually work? Sunlight, which consists of individual light quanta called photons, is absorbed in the solar cell. The photons transfer their energy to electrons, lifting them to higher-energy states where they are free to move. The free electrons are then extracted at electrical contacts and converted into usable electrical energy.
The efficiency of a solar cell thus depends crucially on how effectively these short-lived charge carriers can travel through the material to reach the contacts before decaying. To further optimize solar cells strategically, it is essential to understand exactly how this transport takes place, including the pathways electrons take and what hinders their movement.
This challenging task has now been accomplished by researchers at the University of Regensburg led by Prof. Dr. Rupert Huber with a new type of ultrafast microscope using tailor-made samples from Prof. Dr. Michael Johnston (Oxford University). The team managed to generate free electrons and track their diffusion on ultrashort time scales. Their findings are published in the journal Nature Photonics.
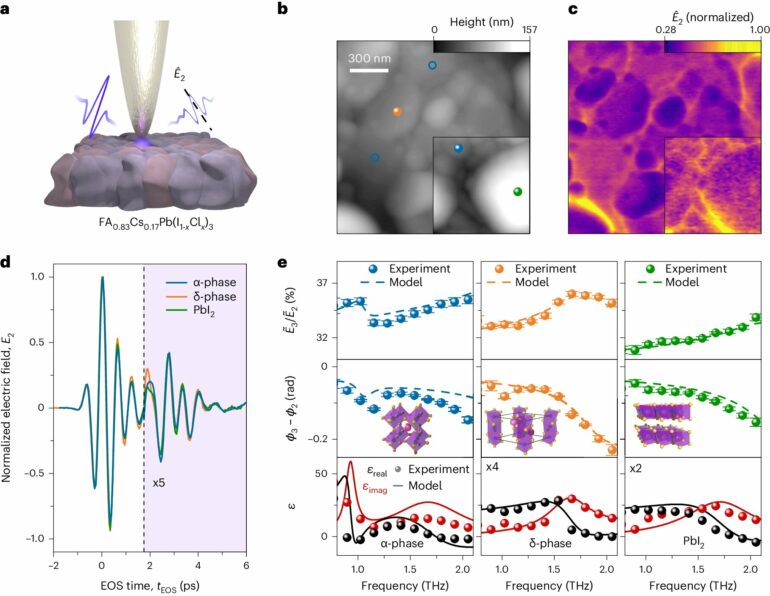
In perovskite solar cells, this constitutes a particular challenge, as they are not homogeneous but consist of many small grains that are only hundreds of nanometers (a billionth of a meter) in size. Moreover, these nanocrystals are not identical throughout the sample; they can exist at room temperature in one of two different atomic structures, only one of which is suitable for use in solar cells. Therefore, it is crucial to pinpoint the exact location and crystalline structure under investigation.
To this end, the researchers used a microscope that can zoom into the nanoscale, making it possible to measure these nanocrystals one by one. Simultaneously, optical methods can be used to ascertain that they are positioned on a crystallite with the correct atomic structure.
“We make the atoms in the nanocrystallites vibrate. Depending on the arrangement of the atoms, these vibrations create distinct signatures in the scattered light, akin to a fingerprint. This enables us to precisely determine how the atoms are arranged in the respective crystallites,” explains Martin Zizlsperger, first author of the publication.
Once the team knew the exact shape and crystal structure of the nanoislands, they illuminated the sample with a short light pulse, which excited electrons into mobile states—this is exactly what happens when the sun shines over a solar cell. The researchers were then able to measure the subsequent movement of the charges with a second laser pulse.
“Put very simply, the charges behave like a mirror. If these charges now move downward from our measurement point, for instance, the second laser pulse is reflected later. We can then reconstruct the exact movement of the charges based on this miniscule time delay of just a few femtoseconds, where one femtosecond is the millionth part of a billionth of a second,” explains co-author Svenja Nerreter.
This made it possible to observe exactly how the excited electrons move through the labyrinth of different crystallites. In particular, the researchers were also able to investigate the technically relevant movement in the direction perpendicular to the surface of the solar cell after excitation.
The results were surprising: Although the material consists of many different nanocrystals, the vertical charge transport on the nanometer length scale remains unaffected by irregularities in nanocrystallites’ shapes. This could be a possible reason for the success of perovskite solar cells.
However, when the researchers investigated larger regions on the scale of several hundred micrometers, they found differences in charge transport efficiency among micrometer-sized regions consisting of hundreds of small nanocrystallites, with some regions being more efficient than others.
These local hotspots could be of great importance for the development of new solar cells. The researchers’ novel measurement method can provide direct insight into the distribution and efficiency of the individual regions, marking an important step towards further improving perovskite solar cells.
“Our newly developed method allows us to observe the complex interplay between charge transport, crystal configuration and the shape of the crystallites directly on the nanoscale for the first time. It can therefore be used to further improve perovskite solar cells in a targeted manner,” explains Prof. Huber.
However, the new measurement concept is not limited to solar cells, as the interplay between structure and charge transport is of central importance for a large variety of modern applications.
The breakthrough could also significantly advance the development of ultra-small, high-speed transistors and help unravel one of the greatest mysteries in solid-state physics—high-temperature superconductivity.
More information:
M. Zizlsperger et al, In situ nanoscopy of single-grain nanomorphology and ultrafast carrier dynamics in metal halide perovskites, Nature Photonics (2024). DOI: 10.1038/s41566-024-01476-1
Provided by
Universität Regensburg
Citation:
Small steps for electrons—big steps for the future? Ultrafast microscope reveals electron pathways in solar cells (2024, July 17)