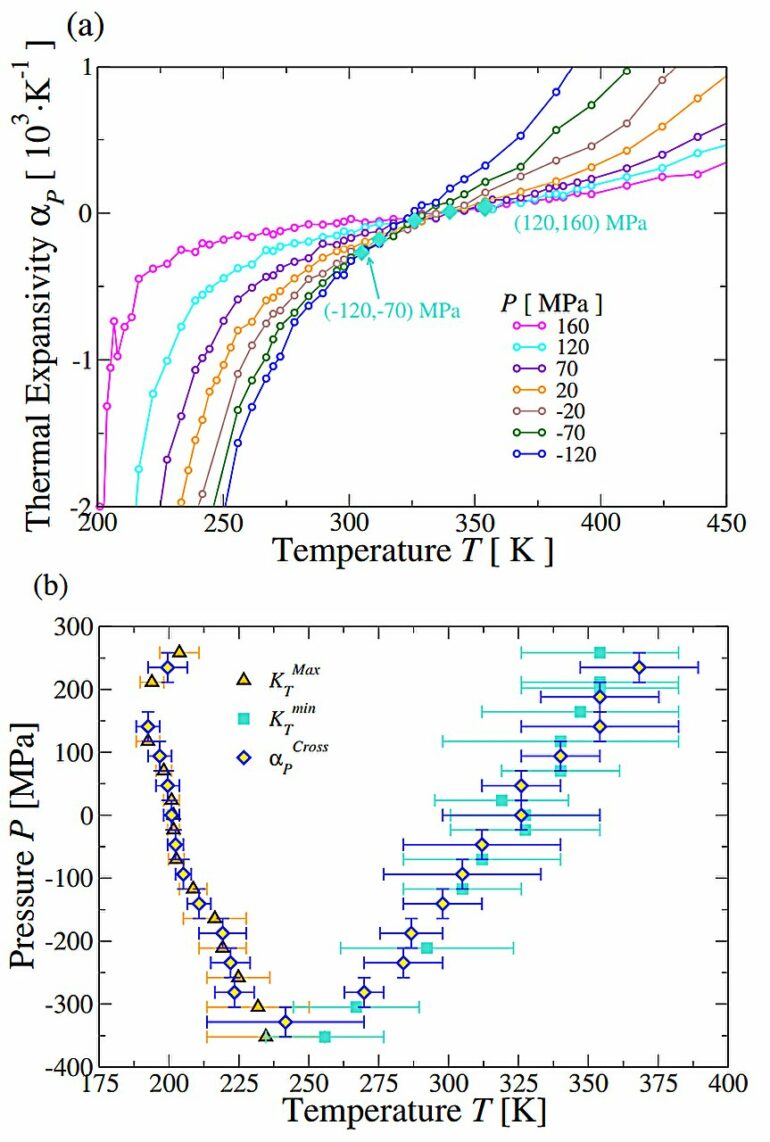
Water, a molecule essential for life, has unusual properties—known as anomalies—that define its behavior. However, there are still many enigmas about the molecular mechanisms that would explain the anomalies that make the water molecule unique. Deciphering and reproducing this particular behavior of water in different temperature ranges is still a major challenge for the scientific community.
Now, a study presents a new theoretical model capable of overcoming the limitations of previous methodologies to understand how water behaves in extreme conditions. The paper, featured on the cover of The Journal of Chemical Physics, is led by Giancarlo Franzese and Luis Enrique Coronas, from the Faculty of Physics and the Institute of Nanoscience and Nanotechnology of the University of Barcelona (IN2UB).
The study not only broadens our understanding of the physics of water, but also has implications for technology, biology and biomedicine, in particular for addressing the treatment of neurodegenerative diseases and the development of advanced biotechnologies.
The CVF model: Better understanding the physics of water
The study, which results from the doctoral thesis that Coronas presented in 2023 at the Faculty of Physics of the UB, shows a new theoretical model that responds to the acronym CVF (the initials of the surnames of the researchers Coronas, Oriol Vilanova and Giancarlo Franzese). The new CVF model is reliable, efficient, scalable and transferable, and incorporates ab initio quantum calculations that accurately reproduce the thermodynamic properties of water under different conditions.
By applying the new theoretical framework, the study reveals that “there is a critical point between two liquid forms of water, and this critical point is the origin of the anomalies that make water unique and essential for life, as well as for many technological applications,” says Professor Giancarlo Franzese, from the Statistical Physics Section of the Department of Condensed Matter Physics.
“Although this conclusion has already been reached in other water models, none of them have the specific characteristics of the model we have developed in this study,” says Franzese.
“However, the CVF model does this because it incorporates results from initial quantum calculations of interactions between molecules. These interactions, known as many-body problems, go beyond classical physics and are due to the fact that water molecules share electrons in a way that is difficult to measure experimentally,” says Franzese.
According to the study, “fluctuations in density, energy and entropy in water are regulated by these quantum interactions, with effects ranging from the nanometer to the macroscopic scale,” says researcher Coronas.
“For example,” Coronas continues, “water regulates the exchange of energy and molecules, as well as the state of aggregation of proteins and nucleic acids in cells. Defects in these processes are suspected to cause serious diseases such as Alzheimer’s, Parkinson’s and amyotrophic lateral sclerosis. Understanding how water fluctuations contribute to these processes could therefore be key to finding treatments for these diseases.”
Fostering the development of new biotechnologies
The CVF model also offers new advantages that allow calculations to be performed where other models fail, either because they are computationally too heavy or because they deviate significantly from experimental results.
In the field of technological development, some laboratories are developing biotechnologies to replace muscles (mechanical actuators) that take advantage of the quantum interactions of water; water-based memristors to create memory devices (with a capacity millions of times greater than current ones), or the application of graphene sponges that separate water from impurities thanks to fluctuations in the density of water in nanopores.
There are also implications for understanding the physics of water. “This model can reproduce the properties of liquid water at virtually all temperatures and pressures found on our planet, although it deviates at extreme conditions reached in laboratories,” say the experts.
“This shows that effects not included in the model—nuclear quantum effects—are also important at these extreme pressures and temperatures. Thus, the limitations of the model guide us where to improve in order to arrive at a definitive formulation of the model,” they conclude.
More information:
Luis Enrique Coronas et al, Phase behavior of metastable water from large-scale simulations of a quantitatively accurate model near ambient conditions: The liquid–liquid critical point, The Journal of Chemical Physics (2024). DOI: 10.1063/5.0219313
Provided by
University of Barcelona
Citation:
Theoretical model explains the anomalous properties of water in extreme conditions (2024, November 14)