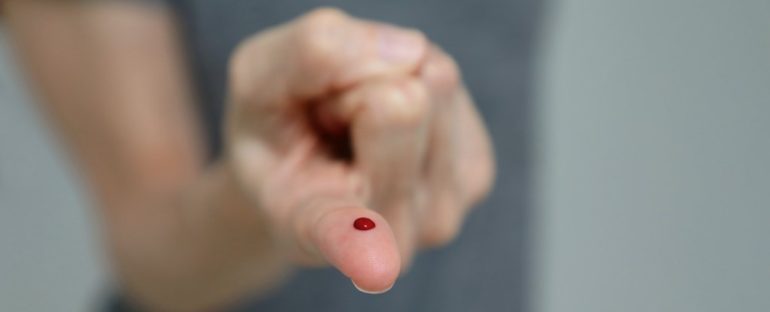
A rapid finger-prick test designed to show whether a person has previously been infected with SARS-CoV-2 is significantly less accurate than earlier research suggested, scientists report in a new study.
The AbC-19 Rapid Test, developed for use by healthcare professionals in the UK and EU, looks for antibodies against the virus in a small drop of blood from a finger-prick, and can show results in just 20 minutes, without needing specialised lab equipment.
The idea is that healthcare workers can quickly and easily run the test in public at points of care, and receive results on the spot to provide insight into how many people in the community have antibodies against SARS-CoV-2 – a strong selling point that led the UK government to order a million of the test devices for £75 million (almost US $100 million).
That order was also guided by positive results of an “extensive validation study” funded by the UK-Rapid Test Consortium – a body that represents commercial companies, including Abingdon Health and Omega Diagnostics, which developed AbC-19.
That study, led by researchers from Ulster University in Northern Ireland, is publicly available but is still awaiting peer review.
It found, effectively, that the AbC-19 Rapid Test would give no false positive results, with a specificity of 100 percent. Specificity is the ability to correctly identify a true negative sample, rather than give a false negative.
The Ulster study also found that the test’s sensitivity was 97.7 percent. Sensitivity is the ability to correctly identify a true positive sample.
Now, however, a new independent study of AbC-19 has found significantly different results in terms of the finger-prick test’s accuracy.
A team of scientists from the Universities of Bristol, Cambridge, and Warwick analysed blood samples from 2,847 key workers (healthcare workers and first responders) – 268 of which had previously delivered a positive PCR result for COVID, while 2,579 had an unknown previous infection status.
In addition, they tested samples from 1,995 pre-pandemic blood donors (known negatives from before the coronavirus pandemic).
The results of the new study suggest AbC-19’s specificity is 97.9 percent (not 100 percent, as the Ulster study claimed), and its sensitivity is 92.5 percent (based on PCR confirmed cases) but can drop as low as 84.7 percent in cases where prior infection status is entirely unknown.
The differences between the two studies likely reflect differences in how the two groups tested the AbC-19 device, but it’s being suggested that the Ulster research didn’t provide as clear a picture as it might have of the test’s accuracy.
“[The Ulster study] chose as known positives people who had already tested positive for antibodies to SARS-CoV-2 proteins in three other assays and chose as known negatives people who tested negative in the same three assays,” two clinical experts, Dipender Gill and Mark J Ponsford, write in a commentary article on the new study’s findings.
“Such a relatively extreme choice of reference standards likely overestimated the accuracy of the AbC assay, owing to a well known phenomenon called spectrum bias.”
Extrapolating further, the independent study – led by first author Ranya Mulchandani from the UK Field Epidemiology Training Program – found one in five key workers testing positive with AbC-19 would be a false positive, in a scenario where 10 percent of the tested population had been infected with SARS-CoV-2.
While no test is ever perfect, the reported reduced accuracy of the AbC-19 test is something people should be aware of, researchers say.
“These new data are very useful at a public health level,” says infectious diseases researcher Eleanor Riley from the University of Edinburgh, who wasn’t involved with the studies.
“If we know how many cases the test is missing, and how many it is wrongly calling positive, we can adjust our population estimates of prior infection accordingly.”
For its part, the UK Department for Health & Social Care (DHSC) insists the new findings aren’t a problem for AbC-19’s intended use – which is in monitoring prior infections in the community, from a healthcare level, and not diagnosing current COVID-19 infections in members of the public.
“This report shows these tests are approved for use in surveillance studies, which is what they were purchased for,” a statement reads.
“They were never intended for, and have never been issued for widespread public use and it is misleading and unnecessarily inflammatory to purposefully ignore this fact in the report.”
Nonetheless, the fallout from these new revelations may be considerable. There are allegations that the UK government delayed the findings of the new study, and already legal proceedings have commenced against the government in relation to the tests.
The findings are reported in The BMJ.