Researchers have developed a new technology which could enable lithium batteries to be replaced with more sustainable alternatives.
A team at Imperial College London have created a technology which could enable the transition from lithium-ion to sodium-ion batteries. By preparing carbons from lignin, a waste by-product of the paper industry, researchers improved the energy density, sustainability and safety of sodium-ion batteries. The results, published in Energy and Environmental Science, could play a significant role in the global transition to greener energy sources.
Replacing lithium-ion batteries
Lithium-ion batteries are commonly used in many electrical devices, but global lithium resources are rapidly declining and mining operations create a large carbon footprint. Sodium-ion batteries could offer a sustainable alternative which, if optimized, could be used in electric vehicles. However, current models have yet to match lithium’s energy capacity and pose some safety risks.
The key to improving energy capacity in batteries lies in the material and design of anodes, which provide the energy storage function. Despite their ultra-high theoretical capacity, further research has been needed to unlock the potential of batteries made with metallic sodium anodes and address the risks posed by this material’s highly reactive nature.
The Titirici Group in the Department of Chemical Engineering has taken up this challenge and developed a solution by replacing the bulk metals in sodium batteries with sodium-deposited lignin-derived carbon mats.
Corresponding author Professor Magda Titirici said: “It is exciting to see new opportunities for lignin utilization in the battery sector and its potential to develop new sodium-based technologies, which could revolutionize the electric vehicle sector by creating high performance, safe and more sustainable batteries”.
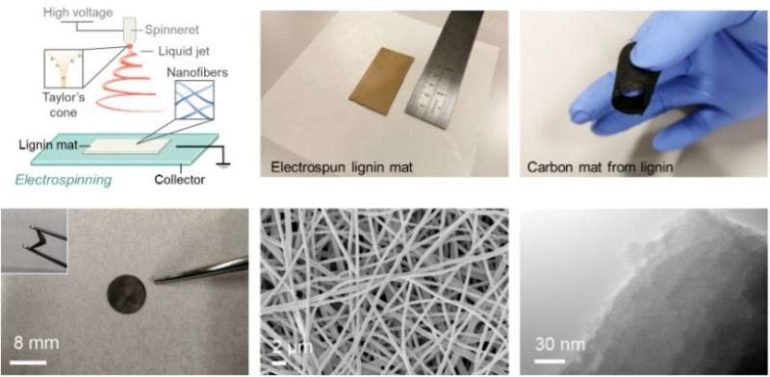
Spinning webs
In this study, lignin mats were produced using a process known as ‘electrospinning’ where lignin fibers are spun in a similar manner to how spiders spin their webs in nature. The fibers were then carbonized to produce numerous defects in the material structure. These defects support an even and stable deposition of metallic sodium, the element responsible for energy storage, resulting in an increased energy capacity.
By combining metallic sodium with specially tailored lignin-based carbon, the energy capacity benefits are retained and utilized while the safety risks associated with a build-up of dendrite—which causes batteries to short-circuit—are reduced.
The process also requires less heat to carbonize the lignin than other methods, which further reduces the carbon footprint of the manufacturing process.
Next steps
Sodium-ion batteries have shown immense promise in the energy field, but their limited energy capacity has so far restricted their widespread uptake. This new technology could enable them to replace lithium-ion batteries on a much wider scale than is currently possible and be used in products as large as electric vehicles.
Co-author Zhen Xu added: “Our research shows the great potential for sodium-ion batteries to play a significant role in a sustainable energy future. Now we hope to work with industry to develop this technology on an industrial scale and explore new applications for sodium-ion batteries.”
The team will now continue to fine-tune the technology, experimenting with different types of cells and carrying out further experiments.
Proliferation of electric vehicles based on high-performance, low-cost sodium-ion battery
More information:
‘Homogenous metallic deposition regulated by defect-rich skeletons for sodium metal batteries’ by Zhen Xu et. al. is published in Energy & Environmental Science.
DOI: doi.org/10.1039/D1EE01346G
Provided by
Imperial College London
Citation:
Scientists develop technology for sustainable next-generation batteries (2021, August 19)
retrieved 21 August 2021
from https://techxplore.com/news/2021-08-scientists-technology-sustainable-next-generation-batteries.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.