Carbon capture, or the isolation and removal of carbon dioxide from the atmosphere during industrial processes like cement mixing or steel production, is widely regarded as a key component of fighting climate change. Existing carbon capture technologies, such as amine scrubbing, are hard to deploy because they require significant energy to operate and involve corrosive compounds.
As a promising alternative, researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed carbon capture systems that use molecules called quinones, dissolved in water, as their capturing compounds.
A study in Nature Chemical Engineering provides critical insights into the mechanisms of carbon capture in these safer, gentler, water-based electrochemical systems, paving the way for their further refinement.
Led by former Harvard postdoctoral fellow Kiana Amini, now an assistant professor at University of British Columbia, the study outlines the detailed chemistry of how an aqueous, quinone-mediated carbon capture system works, showcasing the interplay of two types of electrochemistry that contribute to the system’s performance.
The study’s senior author is Michael J. Aziz, the Gene and Tracy Sykes Professor of Materials and Energy Technologies at SEAS. Aziz’ lab previously invented a redox flow battery technology that uses similar quinone chemistry to store energy for commercial and grid applications.
Quinones are abundant, small organic molecules found in both crude oil and rhubarb that can convert, trap, and release CO2 from the atmosphere many times over. Through lab experiments, the Harvard team knew that quinones trap carbon in two distinct ways.
These two processes happen simultaneously, but the researchers have been unsure of each one’s contributions to overall carbon capture—as if their experimental electrochemical device were a black box.
This study opens the box.
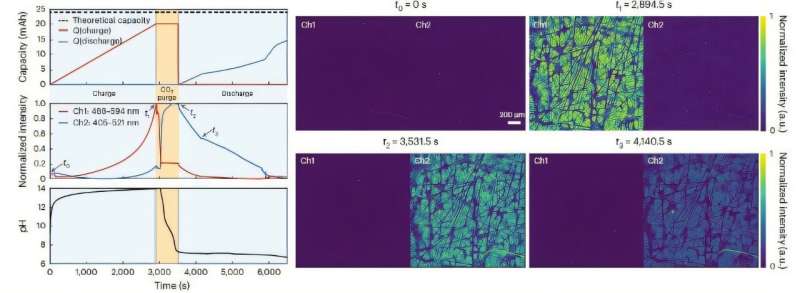
Fluorescence images taken from inside an operating electrochemical CO2 capture/release flow cell, alongside measured data showing capacity, normalized fluorescence intensity, and pH changes over time. © Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00153-y
“If we are serious about developing this system to be the best it can be, we need to know the mechanisms that are contributing to the capture, and the amounts … we had never measured the individual contributions of these mechanisms,” Amini said.
One of the ways dissolved quinones trap carbon is a form of direct capture, in which quinones receive an electrical charge and undergo a reduction reaction that gives them affinity to CO2. The process allows quinones to attach to the CO2 molecules, resulting in chemical complexes called quinone-CO2 adducts.
The other way is a form of indirect capture in which the quinones are charged and consume protons,which increases the solution’s pH. This allows CO2 to react with the now-alkaline medium to form bicarbonate or carbonate compounds.
The researchers devised two real-time experimental methods for quantifying each mechanism. In the first, they used reference electrodes to measure voltage signature differences between the quinones and resulting quinone-CO2 adducts.
In the second, they used fluorescence microscopy to distinguish between oxidized, reduced, and adduct chemicals and quantified their concentrations at very fast time resolutions. This was possible because they discovered that the compounds involved in quinone-mediated carbon capture have unique fluorescence signatures.
“These methods allow us to measure contributions of each mechanism during operation,” Amini said. “By doing so, we can design systems that are tailored to specific mechanisms and chemical species.”
The research advances understanding of aqueous quinone-based carbon capture systems and provides tools for tailoring designs to different industrial applications. While challenges remain, such as oxygen sensitivity that can hinder performance, these findings open new avenues for investigation.
More information:
Kiana Amini et al, In situ techniques for aqueous quinone-mediated electrochemical carbon capture and release, Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00153-y
Provided by
Harvard John A. Paulson School of Engineering and Applied Sciences
Citation:
Exploring quinone-based carbon capture: A promising path to safer CO₂ removal (2025, January 10)