Metal production is responsible for 10% of global CO2 emissions, with iron production emitting two tons of CO2 for every ton of metal produced, and nickel production emitting 14 tons of CO2 per ton and even more, depending on the ore used.
These metals form the foundation of alloys that have a low thermal expansion, called Invar. They are critical for the aerospace, cryogenic transport, energy and precision instrument sectors.
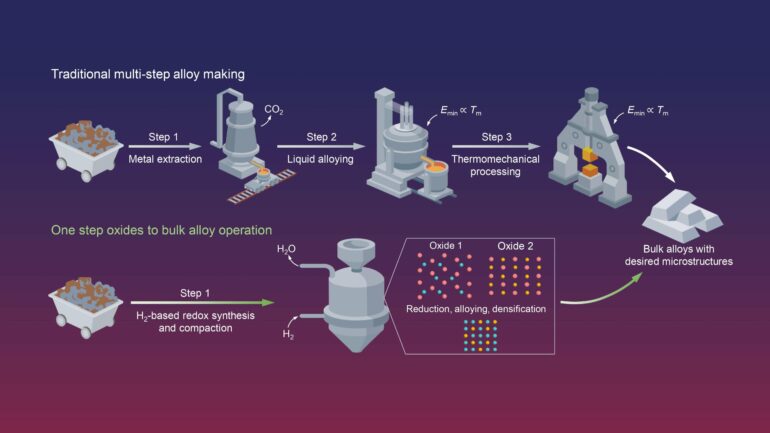
Recognizing the environmental toll, scientists at the Max Planck Institute for Sustainable Materials (MPI-SusMat) have now developed a new method to produce Invar alloys without emitting CO2 while saving a vast amount of energy—achieving this in a single-step process that integrates metal extraction, alloying, and thermomechanical processing into a single reactor and process step.
Their approach dissolves some of the classical boundaries between extractive and physical metallurgy, inspiring direct conversion from oxides to application-worthy products in one single solid-state operation. Their findings are published in the journal Nature.
One-step-metallurgy saves energy and CO2
“We asked ourselves: can we produce an alloy with near-optimized microstructure-property combination directly from ores or oxides with zero CO2 emission?” says Dr. Shaolou Wei, Humboldt research fellow at MPI-SusMat and first author of the publication.
Conventional alloy production is typically a three-step process: first, reducing ores to their metallic form, then mixing liquified elements to create the alloy, and finally applying thermomechanical treatments to achieve the desired properties. Each of these steps is energy-intensive and relies on carbon as both an energy carrier and a reducing agent, resulting in significant CO2 emissions.
“The key idea is to understand the thermodynamics and kinetics of each element and use oxides with similar reducibility and mixability at around 700°C,” Dr. Wei continues, “This temperature is far below the bulk melting point, which still allows us to extract metals from their oxide states and mix into alloys via one single solid-state process step without reheating.”
Unlike conventional methods where ores are reduced using carbon, which results in carbon-contaminated metals, the team’s new method uses hydrogen as the reducing agent. “Using hydrogen instead of carbon brings four key advantages,” explains Professor Dierk Raabe, managing director at MPI-SusMat and corresponding author of the study.
“First, the hydrogen-based reduction only produces water as a byproduct, meaning zero CO2 emissions. Second, it yields pure metals directly, eliminating the need to remove carbon from the final product, thus saving time and energy. Third, we do the process at comparably low temperatures, in the solid state. Fourth, we avoid the frequent cooling and reheating characteristics of conventional metallurgical processes.”
The resulting Invar alloys produced using this technique not only match the low thermal expansion properties of conventionally produced Invar alloys, but also offer superior mechanical strength due to the refined grain size naturally inherited from the process.
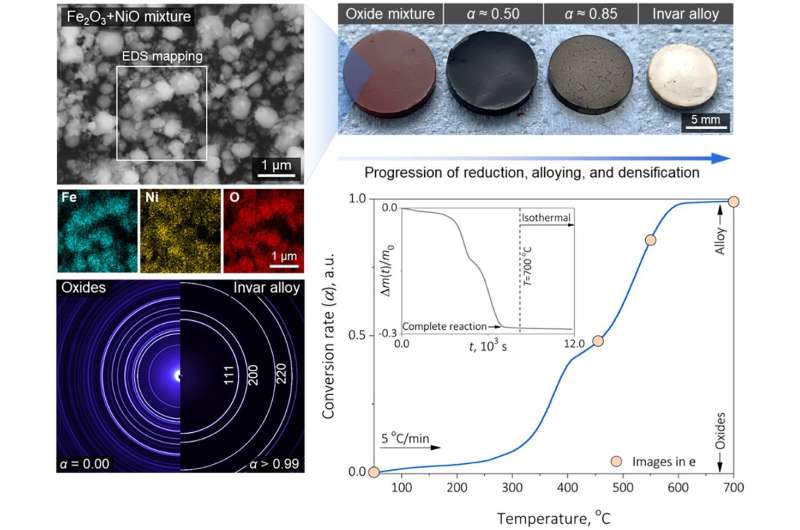
Synthesis of Fe-36Ni invar alloy from iron and nickel oxides. © Nature (2024). DOI: 10.1038/s41586-024-07932-w
Upscaling to industrial dimensions
The Max Planck scientists have demonstrated that producing Invar alloys through a fast, carbon-free, and energy-efficient process is not only possible but highly promising. However, scaling this method to meet industrial demands presents three key challenges:
First, while the researchers used pure oxides for a proof-of-concept study, industrial applications would likely involve conventional, impurity-laden oxides. This introduces the need to adapt the process to handle less refined materials while maintaining alloy quality.
Second, the use of pure hydrogen in the reduction process, though effective, is costly for large-scale operations. The team is now conducting experiments with lower hydrogen concentrations at elevated temperatures to find an optimal balance between hydrogen use and energy costs, making the process more economically viable for industry.
Third, while the current method uses pressure-free sintering, producing finely coarsened bulk materials on an industrial scale would likely require the addition of pressing steps. Incorporating mechanical deformation into the same process could further enhance the material’s structural integrity while keeping the production streamlined.
Looking ahead, the versatility of this one-step process opens up new possibilities. Since iron, nickel, copper, and cobalt can all be processed this way, high-entropy alloys could be the next focus. These alloys, known for their ability to maintain unique properties across a broad range of compositions, hold the potential for developing new materials, such as soft magnetic alloys, ideal for high-tech applications.
Another promising direction could be the use of metallurgical waste instead of pure oxides. By removing impurities from waste materials, this approach could transform industrial byproducts into valuable feedstock, further enhancing the sustainability of metal production.
By eliminating the need for high temperatures and fossil fuels, this one-step hydrogen-based process could drastically reduce the environmental footprint of alloy production, paving the way for a greener, more sustainable future in metallurgy.
More information:
Shaolou Wei et al, One step from oxides to sustainable bulk alloys, Nature (2024). DOI: 10.1038/s41586-024-07932-w
Provided by
Max Planck Society
Citation:
Innovating alloy production: A single step from ores to sustainable metals (2024, September 19)