Lithium-ion batteries have a lot of advantages. They charge quickly, have a high energy density, and can be repeatedly charged and discharged.
They do have one significant shortcoming, however: they’re prone to short-circuiting. This occurs when a connection forms between the two electrodes inside the cell. A short circuit can result in a sudden loss of voltage or the rapid discharge of high current, both causing the battery to fail. In extreme cases, a short circuit can cause a cell to overheat, start on fire, or even explode.
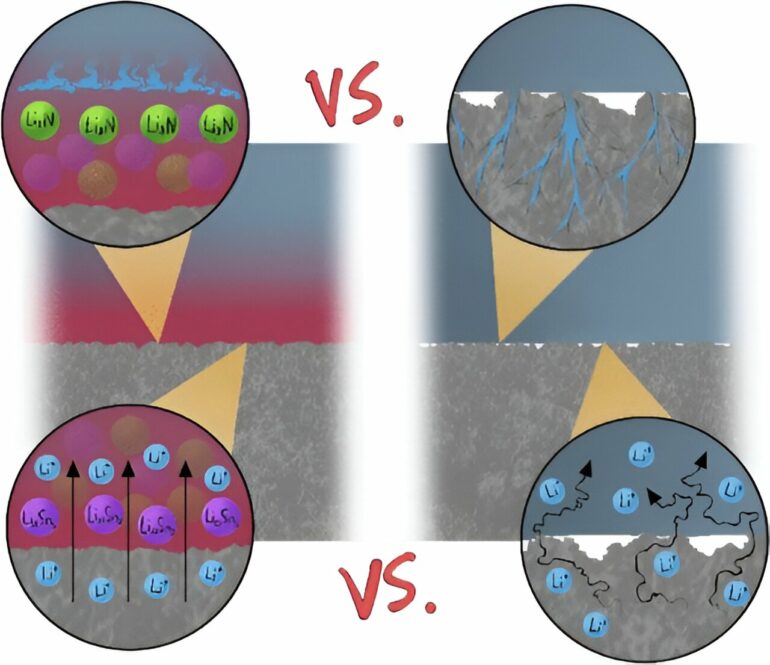
A leading cause of short circuits are rough, tree-like crystal structures called dendrites that can form on the surface of one of the electrodes. When dendrites grow all the way across the cell and make contact with the other electrode, a short circuit can occur.
Using the Canadian Light Source (CLS) at the University of Saskatchewan (USask), researchers from the University of Alberta (UAlberta) have come up with a promising approach to prevent formation of dendrites in solid-state lithium-ion batteries. They found that adding a tin-rich layer between the electrode and the electrolyte helps spread the lithium around when it’s being deposited on the battery, creating a smooth surface that suppresses the formation of dendrites.
The results are published in the journal ACS Applied Materials and Interfaces. The team also found that the cell modified with the tin-rich structure can operate at a much higher current and withstand many more charging-discharging cycles than a regular cell.
Researcher Lingzi Sang, an assistant professor in UAlberta’s Faculty of Science (Chemistry), says the CLS played a key role in the research.
“The HXMA beamline enabled us to see at a material’s structural level what was happening on the surface of the lithium in an operating battery,” says Sang. “As a chemist, what I find the most intriguing is we were able to access the exact tin structure that we introduced to the interface which can suppress dendrites and fix this short-circuiting problem.”
In a related paper the team published earlier this year, they showed that adding a protective layer of tin also suppressed the formation of dendrites in liquid-electrolyte-based lithium-ion batteries.
This novel approach holds considerable potential for industrial applications, according to Sand. “Our next step is to try to find a sustainable, cost-effective approach to applying the protective layer in battery production,” adds Sang.
More information:
Xiang You et al, Dual-Component Interlayer Enables Uniform Lithium Deposition and Dendrite Suppression for Solid-State Batteries, ACS Applied Materials & Interfaces (2024). DOI: 10.1021/acsami.4c05227
Provided by
Canadian Light Source
Citation:
Adding thin layer of tin prevents short-circuiting in lithium-ion batteries (2024, July 31)