Many owners of electric vehicles worry about how effective their battery will be in very cold weather. Now a new battery chemistry may have solved that problem.
In current lithium-ion batteries, the main problem lies in the liquid electrolyte. This key battery component transfers charge-carrying particles called ions between the battery’s two electrodes, causing the battery to charge and discharge. But the liquid begins to freeze at sub-zero temperatures. This condition severely limits the effectiveness of charging electric vehicles in cold regions and seasons.
To address that problem, a team of scientists from the U.S. Department of Energy’s (DOE) Argonne and Lawrence Berkeley National Laboratories has developed a fluorine-containing electrolyte that performs well even in sub-zero temperatures.
The research appears in Advanced Energy Materials.
“Our team not only found an antifreeze electrolyte whose charging performance does not decline at minus 4 degrees Fahrenheit, but we also discovered, at the atomic level, what makes it so effective,” said Zhengcheng “John” Zhang, a senior chemist and group leader in Argonne’s Chemical Sciences and Engineering division.
This low-temperature electrolyte shows promise of working for batteries in electric vehicles, as well as in energy storage for electric grids and consumer electronics like computers and phones.
In today’s lithium-ion batteries, the electrolyte is a mixture of a widely available salt (lithium hexafluorophosphate) and carbonate solvents such as ethylene carbonate. The solvents dissolve the salt to form a liquid.
When a battery is charged, the liquid electrolyte shuttles lithium ions from the cathode (a lithium-containing oxide) to the anode (graphite). These ions migrate out of the cathode, then pass through the electrolyte on the way into the anode. While being transported through the electrolyte, they sit at the center of clusters of four or five solvent molecules.
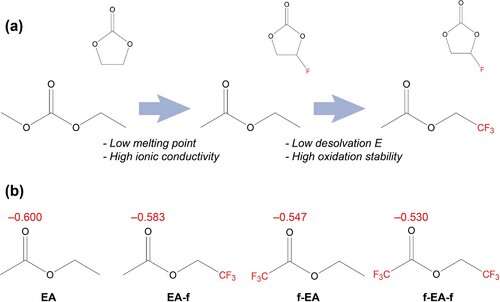
a) Scheme of solvent design transition from carbonates to fluorinated esters. b) Atomic charge analysis of carbonyl groups in EA, EA-f, f-EA, and f-EA-f. © Advanced Energy Materials (2023). DOI: 10.1002/aenm.202204182
During the initial few charges, these clusters strike the anode surface and form a protective layer called the solid-electrolyte interphase. Once formed, this layer acts like a filter. It allows only the lithium ions to pass through the layer while blocking the solvent molecules. In this way, the anode is able to store lithium atoms in the structure of the graphite on charge. Upon discharge, electrochemical reactions release electrons from the lithium that generate electricity that can power vehicles.
The problem is that in cold temperatures, the electrolyte with carbonate solvents begins to freeze. As a result, it loses the ability to transport lithium ions into the anode on charge. This is because the lithium ions are so tightly bound within the solvent clusters. Hence, these ions require much higher energy to evacuate their clusters and penetrate the interface layer than at room temperature. For that reason, scientists have been searching for a better solvent.
The team investigated several fluorine-containing solvents. They were able to identify the composition that had the lowest energy barrier for releasing lithium ions from the clusters at sub-zero temperature. They also determined at the atomic scale why that particular composition worked so well. It depended on the position of the fluorine atoms within each solvent molecule and their number.
In testing with laboratory cells, the team’s fluorinated electrolyte retained stable energy storage capacity for 400 charge-discharge cycles at minus 4 F. Even at that sub-zero temperature, the capacity was equivalent to that of a cell with a conventional carbonate-based electrolyte at room temperature.
“Our research thus demonstrated how to tailor the atomic structure of electrolyte solvents to design new electrolytes for sub-zero temperatures,” Zhang said.
The antifreeze electrolyte has a bonus property. It is much safer than the carbonate-based electrolytes that are currently used, since it will not catch fire.
“We are patenting our low-temperature and safer electrolyte and are now searching for an industrial partner to adapt it to one of their designs for lithium-ion batteries,” Zhang said.
In addition to John Zhang, Argonne authors are Dong-Joo Yoo, Qian Liu and Minkyu Kim. Berkeley Lab authors are Orion Cohen and Kristin Persson.
More information:
Dong‐Joo Yoo et al, Rational Design of Fluorinated Electrolytes for Low Temperature Lithium‐Ion Batteries, Advanced Energy Materials (2023). DOI: 10.1002/aenm.202204182
Provided by
Argonne National Laboratory
Citation:
An electric vehicle battery for all seasons (2023, May 17)



