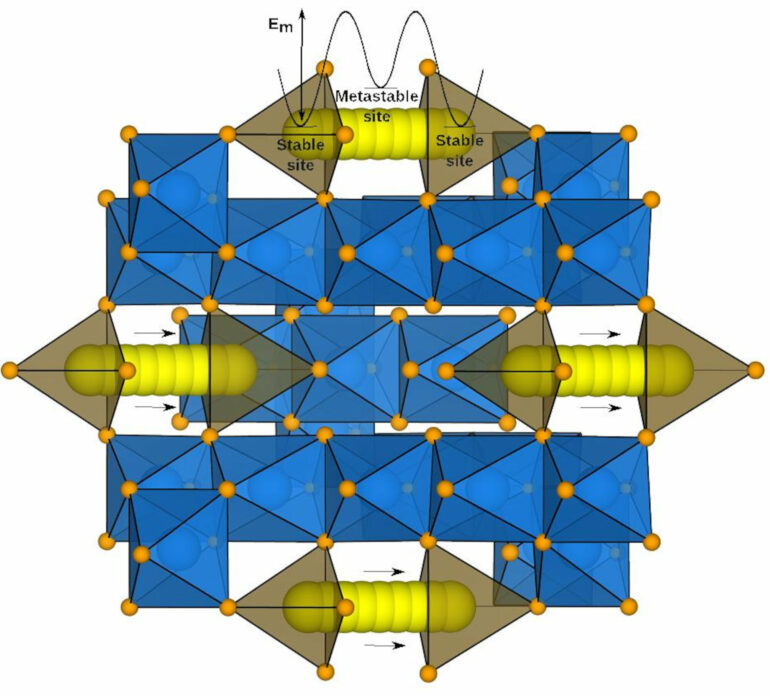
A crucial but poorly-studied parameter that dictates battery performance is the migration barrier. It determines the rate at which ions move through an electrode inside the battery, and ultimately the rate at which it charges or discharges. Because it is hard to measure the migration barrier in the lab, researchers typically use different computer simulations or approximations to quickly predict migration barrier values. However, very few of these simulations have been experimentally verified so far.
In a new study, researchers at the Indian Institute of Science (IISc) and their collaborators comprehensively analyzed widely-used computational techniques, and verified their predictions of the migration barrier values against actual data observed in lab measurements. Based on their analysis, the team proposes a set of robust guidelines to help researchers choose the most accurate computational framework for testing materials that can be used to develop highly efficient batteries in the future.
Lithium-ion batteries, which power mobile phones and laptops, consist of three major components: a solid negative electrode (anode), a solid positive electrode (cathode) and either a liquid or solid electrolyte that separates them. While charging or discharging, lithium ions migrate across the electrolyte, creating a potential difference. “The electrodes in lithium-ion batteries are not 100% solid. Think of them like a sponge. They have ‘pores’ through which a lithium ion has to pass,” explains Sai Gautam Gopalakrishnan, Assistant Professor at the Department of Materials Engineering, IISc, and corresponding author of the paper published in npj Computational Materials.
An important parameter that determines the rate at which the lithium ions penetrate these pores is the migration barrier—the energy threshold that the ions need to overcome to traverse through the electrode. “The lower the migration barrier, the faster you can charge or discharge the battery,” says Reshma Devi, Ph.D. student at the Department of Materials Engineering and first author of the study.
“The same migration barrier value is calculated by one group using one computational technique and another group by using another technique. The values may be equivalent, but we cannot know that for sure,” explains Gopalakrishnan.
Two specific approximations, called Strongly Constrained and Approximately Normed (SCAN) and Generalized Gradient Approximation (GGA), are the most widely used methods to computationally arrive at the migration barrier, but each one has its own disadvantages. “We took nine different materials,” Reshma Devi explains. “We checked which of the approximations come closest to the experimental values for each.”
The team found that the SCAN functional had better numerical accuracy overall, but the GGA calculations were faster. GGA was found to have a reasonable level of accuracy in calculating the migration barrier in certain materials (such as lithium phosphate), and might be a better option if a quick estimation was needed, the researchers suggest.
Such insights can be valuable for scientists who seek to test new materials for their performance before they are adapted for battery-related applications, says Gopalakrishnan. “Suppose you have an unknown material and if you quickly want to see whether this material is useful in your application, then you can use computations to do that, provided you know which computational approximation gives you the closest values. This is useful when it comes to materials discovery.”
The team is also working on developing machine learning tools that can help speed up predictions of migration barriers for a diverse range of materials.
More information:
Reshma Devi et al, Effect of exchange-correlation functionals on the estimation of migration barriers in battery materials, npj Computational Materials (2022). DOI: 10.1038/s41524-022-00837-0
Provided by
Indian Institute of Science
Citation:
Computer simulations aid scientists in gauging battery performance (2022, July 25)