Solid state batteries (SSBs) are an emerging battery technology with high energy densities that could compete with lithium-ion batteries (LIBs), which power a wide range of electronic devices on the market today. In contrast with classic LIBs, SSBs have a solid ‘ceramic’-based electrolyte that separates the anode and cathode inside the battery. In some batteries, this design enables the use of lithium as an anode.
Before SSBs can be commercialized and implemented on a large scale, researchers must identify cost-effective strategies to produce their individual components and develop promising battery cell designs. Researchers at Massachusetts Institute of Technology (MIT) have written a review paper that summarizes recent advances in the field, outlining strategies to process the solid electrolytes and electrolyte/cathode tandems that could be used in future SSB designs.
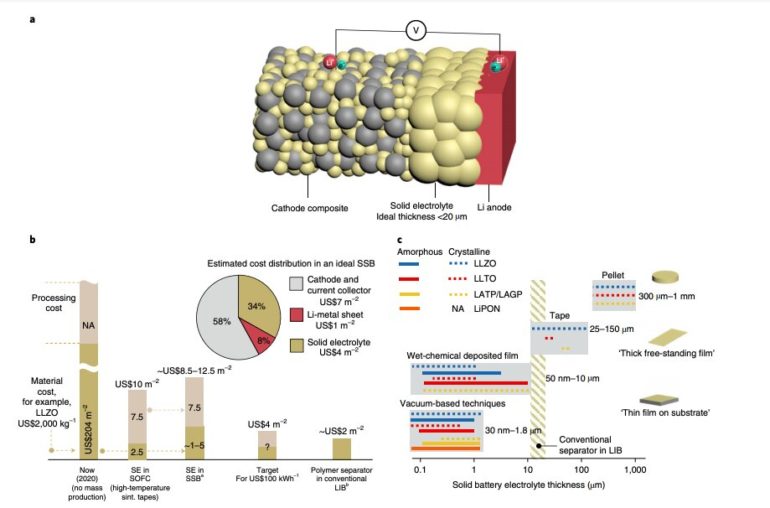
“As most past studies focused on pellet-type solid electrolytes, 75% of the production costs outlined by current cost projections for SSB were greatly overestimated, as they were based on high-temperature classic sintering techniques for solid electrolyte processing,” Moran Balaish, one of the researchers who carried out the study, told TechXplore, via email. “As a result, some projections have concluded that SSB based on oxide solid electrolytes are costly and barely compete with LIBs if cost is the decisive factor. We provide low temperature manufacturing options that impact cell assembly, suggesting that researchers report and reflect not only on classic Arrhenius transport Li+ plots and electrochemical stability windows, but also on the new ‘thermal processing budget.'”
In their paper, Rupp and her colleagues highlight that there are now ample opportunities to manufacture ceramic SSB electrolyte films at low temperatures in the desired size range of 1-20 um. Moreover, they suggest that existing strategies could reduce the costs of SSB production by avoiding expensive co-sinter strategies for producing cathodes and electrolytes.
“For instance, if one avoids high temperature co-sintering in the design and manufacturing of SSB oxide-based cells, this allows the use of less cobalt to produce cathode materials, which could help to avoid geo-socio-political conflicts for resources in the future,” Rupp explained.
In the future, the alternative co-sintering strategies discussed by Rupp and her colleagues could affect the competitiveness of oxide-based Li-based SSBs. In addition, they could pave the way for further research focusing on low-temperature solid batteries for electric vehicles or portable electronics.
“To date, most lab-based research in academia selects the manufacturing of sintered pellets as the way to test materials and assemble cells,” Rupp said. “There are only a few groups researching alternatives, such as the development of tapes and films to adapt realistic and competitive designs for SSBs with thin but robust electrolytes. This has many historic reasons associated with how the field evolved, however, it is disadvantageous that the sintering to pellets limits too strongly the integration of mentioned Cobalt reduced cathodes with an undesirable form factor and high process costs, since more of these cathode materials are simply (by phase diagram) unstable in high temperature co-sintering with the electrolyte component.”
The review paper authored by Rupp and her colleagues ultimately conveys a fairly simple message. More specifically, it highlights the benefits of transitioning to the synthesis of SSB electrolytes in ways that enable dimensions similar to those of classic polymer separators in LIBs. According to the researchers, such a transition would be valuable both to improve the SSBs’ structure and to reduce their costs, while also opening up new possibilities for the integration of cathodes that are not made of cobalt on a much wider scale.
“To our surprise, even though there is the technological need for SSB designs with thin and robust electrolytes, there was still a lack in the field showing most Arrhenius diagrams and electrochemical windows based on data of sintered pellets with mm-sized form factors,” Juan Carlos Gonzalez-Rosillo one of the first authors said.
While several studies have highlighted the potential of SSBs with components that are a few microns thick, so far very few teams have proposed effective strategies to produce these components on a large-scale. In their paper, Rupp and her colleagues propose ways in which this could ultimately be achieved, basing their hypotheses on research evidence gathered over the past few years.
“Some of the questions we asked in our paper are: what methods are suited for developing these components and, importantly, how will these methods affect the thermal processing budget to reduce costs, and provide options to avoid co-sintering for cathode/electrolyte assemblies? Our review is a humble effort to motivate other teams to explore options for the alternative manufacturing of thin and robust SSBs, as well as electrolytes for SSBs,” Rupp added.
In their future research, the researchers plan to focus on two main aspects of SSB development. Firstly, they would like to outline a variety of other strategies that could be used to process cathodes and electrolytes for SSB without relying on co-sintering processes.
“These are challenging and far more time-consuming alternatives then processes based on classic powder-to-pellet or tape routes, as there is a vast parameter field and best densification protocols while keeping stoichiometries of the solid chemistries are not as straightforward,” Rupp explained. “However, if challenges are resolved, these could offer valuable alternative ways of manufacture and this is the door opener towards integration of more Cobalt-reduced cathode materials on the long run.”
Rupp and her colleagues also plan to conduct new studies exploring ways to accelerate the large-scale development and implementation of SSBs. Currently, the design, development and manufacturing of SSB electrolytes in a laboratory setting is estimated to take over 10 years on average. Reducing these components in size factors can take an additional 5-10 years. These times are exceedingly long, highlighting the need for faster processing techniques.
“In our present study, we explore and give a perspective on fast screening and rapid automated processing of ceramic compounds and their chemistries, to test properties and iterate best manufacture routes more quickly to the optimum,” Rupp said. “This is not as straightforward as one may think, since the traditional solid state battery processing routes from academia via powders or sintered compound have some complexity for fast screening and run automated loops. We hope to support our work with concrete examples and analyses on potential methods that are more suited to do fast looping and automation of seeking the best processing condition to make components and cells for future solid state battery designs.”
Solid-state batteries could be made more cleanly by scaling-up flash sintering
More information:
Processing thin but robust electrolytes for solid-state batteries. Nature Energy(2021). DOI: 10.1038/s41560-020-00759-5.
2021 Science X Network
Citation:
Reviewing recent advancements in the development of solid-state batteries (2021, March 19)
retrieved 19 March 2021
from https://techxplore.com/news/2021-03-advancements-solid-state-batteries.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.