Batteries power everything from smartphones to electric vehicles, with their performance hinging on the critical interface between the electrode and electrolyte. Penn State and industry researchers have developed a method to observe this interface at a higher resolution, which could potentially reveal new ways to improve battery efficiency and lifespan.
They published their results in the Journal of the American Chemical Society.
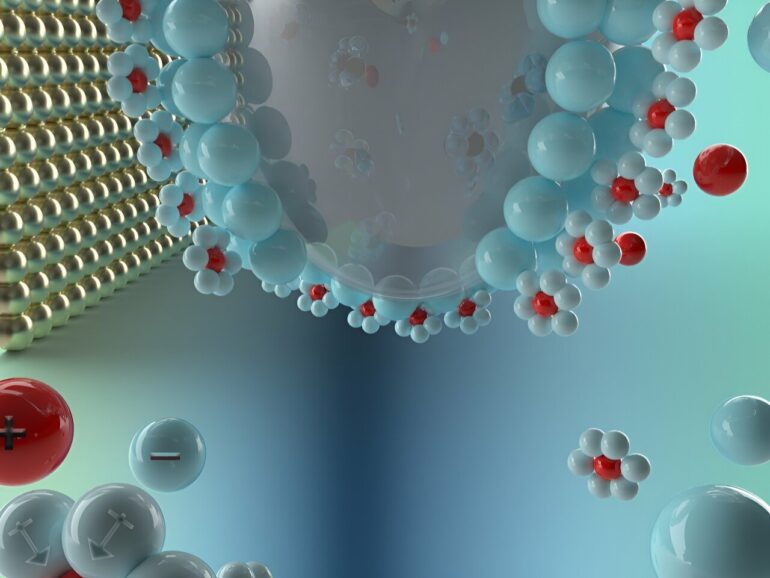
An electrode is a conductor, like a metal rod or plate, that acts as a kind of gateway allowing electricity to enter and leave the battery. There are two types in a battery: Anodes, which are negative electrodes, and cathodes, which are positive ones. Electrolytes are the liquid medium that conducts ions between the anode and cathode, enabling the flow of electrical current.
The electrode–electrolyte interface is the boundary where the solid electrode and liquid electrolyte meet. This interface plays a critical role in the performance of batteries by influencing how ions and solvent molecules accumulate, deplete and transfer charges.
Understanding the behavior of this interface, particularly the electric double layer (EDL), is essential for designing more efficient and durable batteries, according to Jianwei Lai, graduate research assistant in energy and mineral engineering and first author on the study.
“The EDL governs the ion migration and electron transfer that enable electrochemical reactions in batteries,” Lai said. “That’s why studying the double layer is a top priority—it can directly impact battery performance.”
The challenge, however, is that this electrode–electrolyte double layer exists at an ultra-tiny scale and is highly dynamic, changing structure depending on the applied voltage. As the voltage changes, the arrangement of ions and molecules in the layer shifts.
Shifts in the electrode–electrolyte layer can make a battery less efficient, reduce its energy storage and shorten its life, such as when ions get stuck in the wrong spots slowing down the smooth flow of electricity, similar to how traffic jams slow down cars on a highway.
“The EDL is around the nanometer scale, so it’s very hard to characterize,” Lai explained. “And the structure is not static—it’s highly dependent on the applied charge, which makes it very challenging to study directly.”
In the past, scientists have used theoretical models to understand the structure of the EDL. Conventional measurement methods, like voltammetry, traditional electrocapillarity and electrochemical impedance spectroscopy, can provide indirect—but imprecise—clues. This is especially problematic, Lai said, for the more complicated systems in today’s batteries, which include complex salt solutions to help the battery store and release more energy.
To overcome these obstacles, Lai and the team developed a new, improved version of electrocapillarity. This technique measures how the surface tension of the interface changes when a voltage is applied.
The researchers’ new approach uses advanced sensors and equipment to capture rapid changes at the electrode–electrolyte interface. They also developed new analytical methods to assess not just overall interfacial tension but also the specific distribution of ions and potential variations at the interface, providing a clearer and more detailed understanding of the battery performance.
With these measurements, Lai said, they can map the double layer structure and potential profile with unprecedented detail.
“Compared to traditional methods, our high-resolution approach improves the data resolution 50 to 100 times,” Lai said. “We can map out how the double layer looks at each individual voltage or potential. This dynamic nature is something traditional methods just couldn’t capture.”
The researchers used their advanced technique to explore zinc battery electrolytes, an increasingly popular choice for battery production because they are safe and inexpensive. However, figuring out how the surface of the electrolyte interacts with the electrode—and how ions move across this surface—has been difficult, Lai said.
The way ions move at the surface affects how efficiently the battery operates, so understanding this interaction could provide insight into developing better batteries. With their new technique, the team found that more zinc ions gather in the double layer, leading to batteries charging faster and more efficiently.
Their analysis revealed that the zinc ions are guided to the right position by chloride ions, which stick closely to the electrode’s surface, helping guide more zinc ions to the right spot.
“This strategy speeds up charging and makes batteries more efficient by helping zinc ions move faster during charging and discharging,” Lai said. “We can now see how unique this arrangement is and how it improves the overall performance, making the batteries more effective and reliable.”
According to Lai, by having a clearer view of how these parts of the battery work together, scientists can better measure and capture the tiny interactions between the electrode and the electrolyte—allowing them to understand why certain electrolyte components or ion designs might improve battery performance.
Essentially, the technique can serve as a universal platform to understand why the electrolyte works better, which can guide the design of more efficient batteries in the future.
“Understanding this critical interface is essential to help us design better, more efficient and reliable electrolytes for energy storage,” Lai said. “If we know both the individual ion constitution and the interfacial potential profile, then we can really understand how the interface is structured. This is something that was never possible with traditional techniques.”
Armed with this unprecedented level of insight, Lai said he believes they can drive significant advances in electrolyte engineering and, in turn, develop the improved batteries future clean energy-driven technology will demand.
“The modernization of electrocapillarity represents a significant leap forward in the field of electrochemistry,” Lai said. “By providing a direct and precise method to study the electrode–electrolyte interface, this technique will enable researchers to better understand and optimize the critical processes that occur within batteries.
“As the demand for high-performance batteries continues to grow, this research will play a crucial role in driving innovation and improving the energy storage solutions of the future.”
More information:
Jianwei Lai et al, Linking Interfacial Structure and Electrochemical Behaviors of Batteries by High-Resolution Electrocapillarity, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c03791
Provided by
Pennsylvania State University
Citation:
Enhanced electrocapillarity technique advances battery interface analysis (2024, October 7)