Scientists have developed a new method that converts seawater into drinking water that could be useful in disaster zones where there is limited electrical power.
The most popular method for removing salt (sodium chloride) from sea water is reverse osmosis, which uses a porous membrane that allows water molecules through but not salt.
However, this method requires a high pressure and substantial amounts of electricity. The membrane often clogs up, reducing the efficiency of the process.
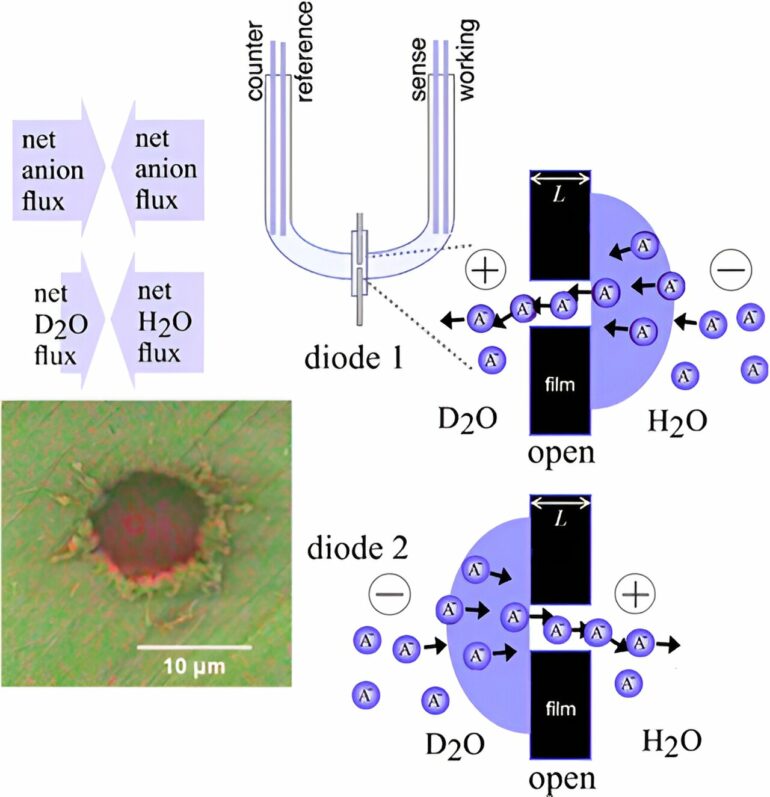
The new technique, developed by a team of scientists from the Universities of Bath, Swansea and Edinburgh, doesn’t use any external pressure but instead uses a small amount of electrical energy to pull chloride ions through the membrane towards a positively charged electrode.
This causes water molecules to be pushed through at the same time as the chloride, a bit like a piston.
Meanwhile, sodium ions remain on the other side of the membrane, attracted to the negatively charged electrode.
The chloride ions are then recycled back into the chamber containing the salt water and the process is repeated, gradually drawing more and more water molecules through.
Professor Frank Marken, from the University of Bath’s Water Innovation Research Centre and Institute for Sustainability led the study, and predicts this could be used on a small scale where drinking water is needed but there is not the infrastructure available, such as in remote areas or disaster zones.
He said, “Currently reverse osmosis uses so much electricity, it requires a dedicated power plant to desalinate water, meaning it is difficult to achieve on a smaller scale.”
“Our method could provide an alternative solution on a smaller scale, and because water can be extracted without any side products, this will save energy and won’t involve an industrial scale processing plant.”
“It could also potentially be miniaturized to use in medical applications such as dosing systems for drugs like insulin.”
So far, the technology is at the proof-of-concept stage, converting only a few milliliters, however the team is now looking for partners for potential collaboration and investment to scale up the process to a liter which will enable them to calculate energy consumption more accurately.
The team would also like to explore other potential applications such as drying processes or recovering water from different sources.
Professor Jan Hoffman, Co-Director of the Water Innovation Research Centre (WIRC) at Bath said, “Zhongkai Li and Frank Marken have developed polymeric materials that can act as a new type of molecular electrical pump for water.”
“I think the discovery can potentially have a revolutionary impact on desalination of seawater and also processes for drying materials and recovering water.”
“Of course, there is still a long way to go to create full scale technology based on the recent discovery, but it definitely looks promising and very innovative compared to existing pumping and desalination technologies.”
Dr. Mariolino Carta from Swansea University commented, “Microporous materials have enormous potential especially in separation and water purification, but also in catalysis.”
“In the future even better materials and processes will be available.”
The research is published in ACS Applied Materials & Interfaces.
More information:
Zhongkai Li et al, Tuning and Coupling Irreversible Electroosmotic Water Flow in Ionic Diodes: Methylation of an Intrinsically Microporous Polyamine (PIM-EA-TB), ACS Applied Materials & Interfaces (2023). DOI: 10.1021/acsami.3c10220
Provided by
University of Bath
Citation:
New method for purifying drinking water could be used in disaster zones (2023, September 21)