Lithium-ion batteries have been at the forefront of energy storage technologies. However, the availability of lithium is limited. Consequently, the growing demand for energy-storage systems has led to the search for low-cost and more accessible materials for rechargeable batteries. Sodium-ion batteries (SIBs) are a promising candidate due to the virtually unlimited sodium (Na) resources in seawater and salt deposits.
Much research has been conducted for improving materials for positive electrodes (cathodes), negative electrodes (anodes), and electrolytes for improving long-cycle stability and achieving a thin solid electrolyte interface (SEI) for SIBs. An SEI is a passive layer formed on the anode surface during the initial charge/discharge cycles, which prevents the anode from degrading due to reactions with the electrolyte.
A well-formed SEI is crucial for battery performance. In this context, hard carbon (HC) has emerged as a promising anode material. Still, its commercialization has been difficult as it forms an uneven, thick, and weak SEI due to increased electrolyte consumption, which lowers charging/discharging stability and reaction speeds.
To address these issues, binders such as carboxymethyl cellulose salts, poly(acrylic acid) derivatives, and poly(vinylidene fluoride) (PVDF) have been used. However, these binders cause slow diffusion of Na ions in the anode, leading to poor rate capability of HC-based SIBs.
To overcome these shortcomings, Professor Noriyoshi Matsumi and Doctoral Course Student Amarshi Patra from the Japan Advanced Institute of Science and Technology (JAIST) developed an HC anode using a poly(fumaric acid) (PFA) binder. Their findings were published in the Journal of Materials Chemistry A on May 10, 2024.
Explaining the benefits of PFA, Prof. Matsumi says, “Unlike conventional poly(acrylic acid) binders, PFA is a high-functional density polymer with carboxylic acid present on all the carbon atoms of the main chain. This enables PFA to improve Na ion diffusion due to the presence of highly concentrated ion hopping sites and to adhere to the electrode more strongly. Additionally, PFA binders offer water solubility and non-toxicity, and its precursor, fumaric acid, is a bio-based polymer.”
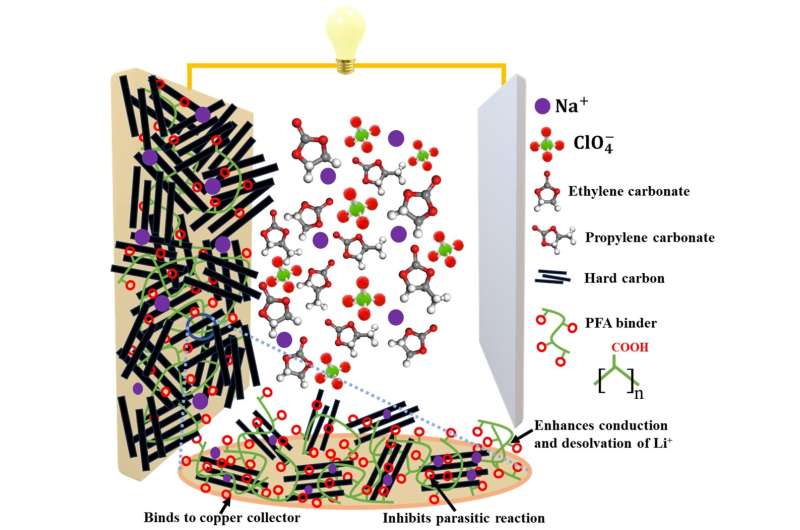
Polyfumaric acid binders improve the diffusion speed of ions and adhesion to the current collector of hard-carbon anodes in sodium-ion batteries, resulting in excellent cycling stability and specific capacity. © Noriyoshi Matsumi from JAIST
The researchers synthesized PFA through hydrolysis of poly(fumarate ester)s. Next, they mixed HC, Super P carbon, and PFA in water to form an aqueous slurry, which was coated onto a copper foil and dried overnight to produce an HC anode. This anode, along with a sodium metal disk as the counter electrode and 1.0 M NaClO4 as the electrolyte, was used to construct an anode-type half-cell.
The researchers conducted a peeling test to test the binder effect on adhesion between electrode components and the copper current collector. Notably, strong adhesion is required for long life of SIBs. The peeling force of the PFA-binder containing HC electrode was found to be 12.5 N, which was significantly higher than poly(acrylic acid)-HC electrodes with 11.5 N and PVDF-HC electrodes with 9.8 N of peeling force.
The anode half-cell was subjected to various electrochemical and battery performance tests. In charging/discharging cycle tests, the anode half-cell showed specific capacities of 288 mAhg-1 and 254 mAhg-1 at current densities of 30 mAg-1 and 60 mAg-1, respectively, significantly better than PVDF and poly(acrylic acid)-type electrodes. It also showed excellent long-cycle stability, retaining 85.4% of its capacity after 250 cycles.
The anode formed a thin SEI and did not show crack formation or exfoliation, which contributed to the enhanced durability of the half-cell. Furthermore, the Na ion diffusion coefficient for the PFA-HC electrode was 1.9 × 10-13 cm2/s, higher than poly(acrylic acid)-HC and PVDF-HC electrodes.
These findings can lead to the development of SIBs with improved electrochemical performance. Looking towards the future, Prof. Matsumi says, “In this polymer material, various structural modifications are possible through different polymer reactions, which can further improve performance. In the future, we aim to conduct joint research with companies for its commercial implementation. Additionally, as a water-soluble and non-toxic binder material that improves durability, it can not only be applied in SIBs but also in a wide range of energy storage devices.”
Overall, this new material can lead to the more widespread use of low-cost energy devices based on SIBs, leading to a more energy-efficient and carbon-neutral society.
More information:
Amarshi Patra et al, Water-soluble densely functionalized poly(hydroxycarbonylmethylene) binder for higher-performance hard carbon anode-based sodium-ion batteries, Journal of Materials Chemistry A (2024). DOI: 10.1039/D4TA00285G
Provided by
Japan Advanced Institute of Science and Technology
Citation:
Researchers develop new electrode binder material for high-performance sodium-ion batteries (2024, May 28)